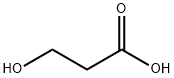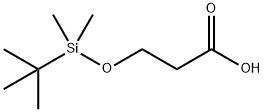
Propanoic acid, 3-[[(1,1-dimethylethyl)dimethylsilyl]oxy]- synthesis
- Product Name:Propanoic acid, 3-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-
- CAS Number:120821-20-7
- Molecular formula:C9H20O3Si
- Molecular Weight:204.34
![3-[(TERT-BUTYLDIMETHYLSILYL)OXY]-1-PROPANAL](/CAS/GIF/89922-82-7.gif)
89922-82-7
85 suppliers
$13.00/100mg
![Propanoic acid, 3-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-](/CAS/20210111/GIF/120821-20-7.gif)
120821-20-7
1 suppliers
inquiry
Yield:120821-20-7 431 mg
Reaction Conditions:
with sodium chlorite;sodium dihydrogenphosphate;2-methyl-but-2-ene in water;tert-butyl alcohol; for 1 h;
Steps:
3.2.2. 1-(Bis(2,2,2-trifluoroethyl)phosphoryl)-4-(tert-butyldimethylsilyloxy)butan-2-one (6)
To a solution of 1,3-propanediol (3 mL, 40 mmol) in THF (60 mL) was added NaH (1.2 g, 60%, 20 mmol) at rt. After stirring for 2.5 h, TBSCl (630 mg, 4.3 mmol) was added and the mixture was stirred overnight before the addition of NaHCO3 solution in MeOH. The solution was then extracted with DCM, washed with water and brine, dried over Na2SO4, filtered, concentrated and purified by flash chromatography (30% EtOAc in hexanes) to afford 2-((tert-butyldimethylsilyl)oxy)ethanol ether (800 mg, 90% based on TBSCl) as a colorless oil. To a solution of this TBS ether (590 mg, 3.11 mmol) in DCM (29 mL) was added NaHCO3 and Dess-Martin periodinane at rt. After stirring for 30 min, the solution was concentrated, diluted with hexane to participate byproduct, filtered and concentrated carefully to give crude 2-((tert-butyldimethylsilyl)oxy)acetaldehyde. A solution of this crude aldehyde in t-BuOH (13 mL) and 2-methyl-2-butene (1.5 mL) was treated with a solution of NaClO2 (500 mg, 5.5 mmol) and NaH2PO4 (1.85 g, 15.4 mmol) in water (12 mL) dropwise. After stirring for 1 h, the reaction was partitioned between brine and DCM. The organic layer was dried over Na2SO4, filtered, concentrated and purified by flash chromatography (30% EtOAc in hexanes) to afford acid 11 (431 mg, 68% over 2 steps) as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 3.91 (2H, t, J = 6.1 Hz), 2.58 (2H, t, J = 6.1 Hz), 0.89 (9H, s), 0.09 (6H, s).
References:
Qi, Jun;Blanden, Adam R.;Bane, Susan;Kingston, David G.I. [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 17,p. 5247 - 5254] Location in patent:experimental part
![methyl 3-[tert-butyl(dimethyl)silyl]oxypropanoate](/CAS/20200331/GIF/448944-56-7.gif)
448944-56-7
5 suppliers
inquiry
![Propanoic acid, 3-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-](/CAS/20210111/GIF/120821-20-7.gif)
120821-20-7
1 suppliers
inquiry
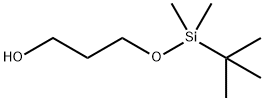
73842-99-6
123 suppliers
$6.00/1g
![Propanoic acid, 3-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-](/CAS/20210111/GIF/120821-20-7.gif)
120821-20-7
1 suppliers
inquiry
dimethyl-](/CAS/20210305/GIF/448944-55-6.gif)
448944-55-6
0 suppliers
inquiry
![Propanoic acid, 3-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-](/CAS/20210111/GIF/120821-20-7.gif)
120821-20-7
1 suppliers
inquiry