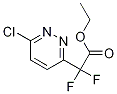
ethyl 2-(6-chloropyridazin-3-yl)-2,2- difluoroacetate synthesis
- Product Name:ethyl 2-(6-chloropyridazin-3-yl)-2,2- difluoroacetate
- CAS Number:1208249-85-7
- Molecular formula:C8H7ClF2N2O2
- Molecular Weight:236.6032

1208249-84-6
2 suppliers
inquiry

1208249-85-7
2 suppliers
inquiry
Yield:1208249-85-7 52.5%
Reaction Conditions:
Stage #1: ethyl 2-(6-chloropyridazin-3-yl)-2-fluoroacetatewith lithium hexamethyldisilazane in tetrahydrofuran at -78; for 0.25 h;
Stage #2: with Selectfluor in tetrahydrofuran;N,N-dimethyl-formamide at -78 - 20;
Steps:
A
To a solution of ethyl 2- (6-chloropyridazin-3-yl)-2-fluoroacetate (36.6 g, 167 mmol) in anhydrous THF (500 rnL) was added lithium hexamethyldisilazide (201 ml, 201 mmol) drop-wise at -78 0C. After 15 minutes, a solution of Selectfluor (71.2 g, 201 mmol) in DMF (183 mL) was added drop- wise. Upon complete addition, the reaction was allowed to warm to ambient temperature over a 30 min period. Saturated NH4Cl (70 mL) was then added, and THF was removed via rotary evaporation. The resulting residue was diluted with water (500 mL), extracted with Et2O (3 x 100 mL), dried with MgSO4, filtered, and concentrated to dryness. The resulting material was purified via MPLC (Hex:EtOAc, 8:2) to provide ethyl 2-(6-chloropyridazin-3- yl)-2,2-difluoroacetate 45D (20.8 g, 88 mmol, 52.5%). 1H NMR (400 MHz, DMSO-J6) δ ppm 1.25 (t, J=7.07 Hz, 3 H) 4.38 (q, J=7.07 Hz, 2 H) 8.26 (d, J=9.09 Hz, 1 H) 8.33 (d, J=8.84 Hz, 1 H). ESI-MS:m/z 237.1 (M+H)+.
References:
WO2010/19899,2010,A1 Location in patent:Page/Page column 165; 167