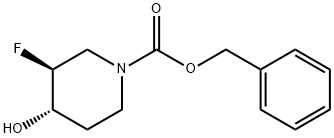
(3S,4S)-benzyl 3-fluoro-4-hydroxypiperidine-1-carboxylate synthesis
- Product Name:(3S,4S)-benzyl 3-fluoro-4-hydroxypiperidine-1-carboxylate
- CAS Number:1209780-78-8
- Molecular formula:C13H16FNO3
- Molecular Weight:253.27

845256-59-9
73 suppliers
$22.00/250mg

1209780-78-8
8 suppliers
inquiry
Yield:-
Reaction Conditions:
with sodium tetrahydroborate in ethanol; for 0.5 h;
Steps:
41
To a stirred solution of 4-trimethylsilanyloxy-3,6-dihydro-2H-pyridine-1-carboxylic acid benzyl ester (5 g, 16.4 mmol) in acetonitrile at 0°C was added 1-(chloromethyl)-4-fluoro-1 ,4- diazoniabicyclo[2.2.2]octane ditetrafluoroborate (6.4 g, 18 mmol). The solution was stirred overnight at room temperature, concentrated, diluted with EtOAc and washed with water. The organics were dried over sodium sulfate, filtered, concentrated, and purified by column chromatography using 40-60% EtOAc in hexanes to give 3-fluoro-4-oxo-pi peridi ne- 1 -carboxyl ic acid benzyl ester (3.1 g) To a stirred solution of 3-fluoro-4-oxo-piperidine-1-carboxylic acid benzyl ester (500 mg, 2 mmol) in ethanol (6 mL) was added sodium borohydride (100 mg, 2.6 mmol). After 0.5 hr, the mixture was concentrated, diluted with EtOAc and washed with water. The organics were dried over sodium sulfate, filtered, concentrated, and purified by column chromatography using 40-70% EtOAc in hexanes to give (+/-)-trans-3-fluoro-4- hydroxy-piperidine-1-carboxylic acid benzyl ester (120 mg).
References:
WO2011/103091,2011,A1 Location in patent:Page/Page column 97
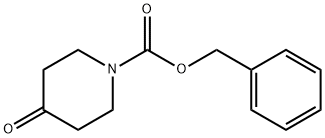
19099-93-5
318 suppliers
$5.00/1g

1209780-78-8
8 suppliers
inquiry

1147998-34-2
14 suppliers
inquiry

1209780-78-8
8 suppliers
inquiry