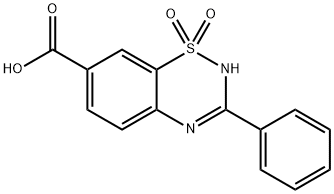
2H-1,2,4-Benzothiadiazine-7-carboxylic acid, 3-phenyl-, 1,1-dioxide synthesis
- Product Name:2H-1,2,4-Benzothiadiazine-7-carboxylic acid, 3-phenyl-, 1,1-dioxide
- CAS Number:1209957-78-7
- Molecular formula:C14H10N2O4S
- Molecular Weight:302.31
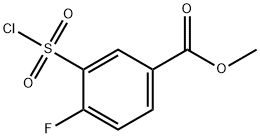
1099660-67-9
15 suppliers
inquiry
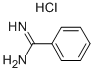
1670-14-0
411 suppliers
$6.00/10g

1209957-78-7
0 suppliers
inquiry
Yield:1209957-78-7 85%
Reaction Conditions:
Stage #1: methyl 3-(chlorosulfonyl)-4-fluorobenzoate;benzamidine monohydrochloridewith potassium hydroxide in dichloromethane;water at 0 - 20;
Stage #2: with hydrogenchloride in dichloromethane;water; pH=1;
Steps:
4.4. One-pot procedure for the preparation of 4H-1,2,4-benzothiadiazine-1,1-dioxides 4c, 4f, and 4g from o-fluoronitrobenzenesulfonyl chlorides 1d, 1g and amidines 2
General procedure: To a stirred solution of equimolar amounts of o-halogenoarylsulfonyl chloride 1 (0.015 mol) and amidine 2 hydrochloride (0.015 mol) in dichloromethane (37 mL) a concentrated water solution of potassium hydroxide (4.2 g, 0.075 mol) was slowly added at 0 °C. The reaction mixture was allowed to stir overnight at room temperature and then acidified with hydrochloric acid to pH 1. The precipitated 4H-1,2,4-benzothiadiazine-1,1-dioxide was filtered, washed with water and dichloromethane, and dried under ambient conditions.
References:
Cherepakha, Artem;Kovtunenko, Vladimir O.;Tolmachev, Andrey;Lukin, Oleg [Tetrahedron,2011,vol. 67,# 34,p. 6233 - 6239] Location in patent:experimental part