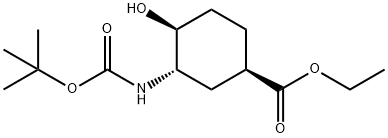
(1R,3S,4S)-3-(Boc-aMino)-4-hydroxy-cyclohexanecarboxylic acid ethyl ester synthesis
- Product Name:(1R,3S,4S)-3-(Boc-aMino)-4-hydroxy-cyclohexanecarboxylic acid ethyl ester
- CAS Number:1210348-16-5
- Molecular formula:C14H25NO5
- Molecular Weight:287.35
![6-Oxabicyclo[3.2.1]octan-7-one, 4-iodo-, (1R,4R,5R)-](/CAS/20210111/GIF/119719-61-8.gif)
119719-61-8
0 suppliers
inquiry

64-17-5
707 suppliers
$10.00/50g

24424-99-5
826 suppliers
$13.50/25G

1210348-16-5
33 suppliers
$286.00/1g
Yield: 67%
Reaction Conditions:
Stage #1:(1R,4R,5R)-4-iodo-6-oxabicyclo[3.2.1]octane-7-one;ethanol with potassium carbonate at 90; for 2 h;Reflux;
Stage #2: with sodium azide;ammonium chloride in N,N-dimethyl-formamide at 80; for 2 h;
Stage #3:di-tert-butyl dicarbonate with hydrogen;palladium 10% on activated carbon in ethyl acetate
Steps:
1.2
(1-2) (1R,3S,4S)-3-[(Tert-butoxycarbonyl)amino]-4-hydroxycyclohexanecarboxylic acid ethyl ester (1R,4R,5R)-4-Iodo-6-oxabicyclo[3.2.1]octan-7-one (25.1 g, 99.6 mmol) was dissolved in ethanol (125 ml). To the solution, potassium carbonate (commercially available) (16.5 g, 119 mmol) was added at 90° C. The mixture was heated to reflux for 2 hours and then allowed to cool to room temperature. The precipitate was filtered off, and the filtrate was concentrated under reduced pressure. The obtained residue was separated into aqueous and organic layers by the addition of ethyl acetate (200 ml) and water (50 ml). Then, the organic layer was washed with water (50 ml) and dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The obtained pale yellow oil (16.7 g) was dissolved in N,N-dimethylformamide (120 ml). To the solution, ammonium chloride (commercially available) (7.77 g, 145 mmol) and sodium azide (commercially available) (9.45 g, 145 mmol) were added at room temperature. The mixture was stirred at 80° C. for 2 hours, then allowed to cool to room temperature, and completely poured to a stirred mixed solution of ethyl acetate (500 ml) and ice water (800 ml). After separation into aqueous and organic layers, the aqueous layer was subjected to extraction with ethyl acetate (2*200 ml), and the organic layers were then combined, washed with water (500 ml) and saturated saline (200 ml), and dried over anhydrous sodium sulfate. To the obtained ethyl acetate solution, di-tert-butyl dicarbonate (commercially available) (27.5 g, 126 mmol) and 10% palladium carbon (commercially available) (1.50 g) were added, and the mixture was stirred overnight under the hydrogen atmosphere. Insoluble matter was filtered off, and the filtrate was then concentrated under reduced pressure. The obtained residue was purified using silica gel column chromatography (silica gel: 400 g, hexane:ethyl acetate=3:1→1:1). Then, the title compound (19.3 g, 67.2 mmol, 67%) was obtained as a white solid by reprecipitation from a hexane-methylene chloride system.1H-NMR (400 MHz, CDCl3) δ: 1.27 (3H, t, J=7.2 Hz), 1.39-1.60 (3H, m), 1.45 (9H, s), 1.85-1.93 (1H, m), 2.08-2.16 (1H, m), 2.29-2.37 (1H, m), 2.61-2.67 (1H, m), 3.31 (1H, br s), 3.39-3.49 (1H, m), 3.54-3.66 (1H, m), 4.10-4.24 (2H, m), 4.54 (1H, br d, J=6.1 Hz).[α]D25: -26.8° (c=1.0, CHCl3).
References:
DAIICHI SANKYO COMPANY, LIMITED US2012/53349, 2012, A1 Location in patent:Page/Page column 11
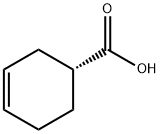
5709-98-8
180 suppliers
$13.00/1g

1210348-16-5
33 suppliers
$286.00/1g
![6-Oxabicyclo[3.2.1]octan-7-one, 4-iodo-, (1R,4R,5R)-](/CAS/20210111/GIF/119719-61-8.gif)
119719-61-8
0 suppliers
inquiry

1210348-16-5
33 suppliers
$286.00/1g
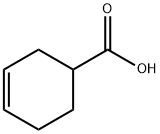
4771-80-6
272 suppliers
$5.00/10g

1210348-16-5
33 suppliers
$286.00/1g