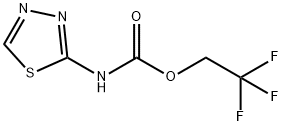
2,2,2-Trifluoroethyl N-(1,3,4-Thiadiazol-2-yl)carbamate synthesis
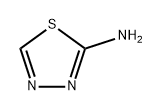
- Product Name:2,2,2-Trifluoroethyl N-(1,3,4-Thiadiazol-2-yl)carbamate
- CAS Number:1211199-35-7
- Molecular formula:C5H4F3N3O2S
- Molecular Weight:227.16
Yield:1211199-35-7 84%
Reaction Conditions:
with triethylamine in acetonitrile at 20 - 30;
Steps:
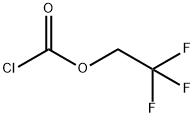
4.3. General procedure for the preparation of trifluoroethyl carbamates (2) from amines and trifluoroethylchloroformate
General procedure: To a stirred solution of 0.3 mol of an amine and 33.4 g (0.33 mol) of triethylamine in 150-200 mL of acetonitrile 50 g (0.3 mol) of trifluoroethylchloroformate was added dropwise. During the addition the temperature of the reaction mixture should be kept below 30 °C. Then the reaction mixture was stirred at room temperature for 1 h, diluted with water, and cooled to 0 °C. The precipitated product was filtered and recrystallized from 2-propanol. All compounds except 2a, b, d, and e remained crystalline solids under ambient condition while the latter turned to oils upon reaching room temperature.
References:
Bogolubsky, Andrey V.;Ryabukhin, Sergey V.;Pipko, Sergey E.;Lukin, Oleg;Shivanyuk, Alexander;Mykytenko, Dmytro;Tolmachev, Andrey [Tetrahedron,2011,vol. 67,# 20,p. 3619 - 3623] Location in patent:experimental part