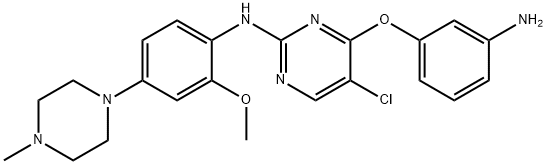
4-(3-aminophenoxy)-5-chloro-N-(2-methoxy-4-(4-methylpiperazin-1- yl)phenyl)pyrimidin-2-amine synthesis
- Product Name:4-(3-aminophenoxy)-5-chloro-N-(2-methoxy-4-(4-methylpiperazin-1- yl)phenyl)pyrimidin-2-amine
- CAS Number:1213269-26-1
- Molecular formula:C22H25ClN6O2
- Molecular Weight:440.93
![5-Chloro-N-[2-methoxy-4-(4-methyl-1-piperazinyl)phenyl]-4-(3-nitrophenoxy)pyrimidin-2-amine](/CAS/20210111/GIF/1213269-25-0.gif)
1213269-25-0
1 suppliers
inquiry

1213269-26-1
19 suppliers
inquiry
Yield:1213269-26-1 76%
Reaction Conditions:
with ammonium chloride;iron in tetrahydrofuran;water at 65; for 4 h;
Steps:
4
4-(3-aminophenoxy)-5-chloro-N-(2-methoxy-4-(4-methylpiperazin-l- yl)phenyl)py rimidin-2-amine 5-chloro-N-(2-methoxy-4-(4-methylpiperazin- 1 -yl)pheny I)- 4-(3-nitrophenoxy) pyrimidin-2-amine (2.00 g, 4.25 mmol) was dissolved in THF (50 mL) and water (50 mL) was added. Iron powder (1.19 g, 21.25 mmol) and ammonium chloride (1.18 g, 21.25mmol) were then added, and the resulting mixture was heated to 650C for 4 hours. The reaction mixture was cooled to room temperature and filtered through celite. The THF was removed in vacuo, and the resulting residue was basified with sodium bicarbonate and extracted with ethyl acetate (50 mL) three times. The organic layer was separated and dried using anhydrous sodium sulfate, concentrated, and purified by flash chromatography with 20:1 dichloromethane-methanol to afford 1.42 g of light yellow solid (76%); MS m/z: 441.93 (M+ 1).
References:
WO2010/129053,2010,A2 Location in patent:Page/Page column 124

122833-04-9
98 suppliers
$10.00/100mg

1213269-26-1
19 suppliers
inquiry
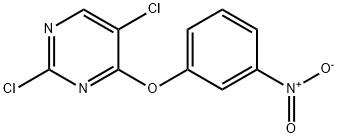
76661-24-0
34 suppliers
inquiry

1213269-26-1
19 suppliers
inquiry
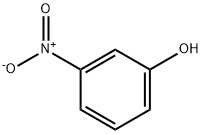
554-84-7
7 suppliers
$12.00/5g

1213269-26-1
19 suppliers
inquiry
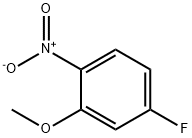
448-19-1
297 suppliers
$5.00/1g

1213269-26-1
19 suppliers
inquiry