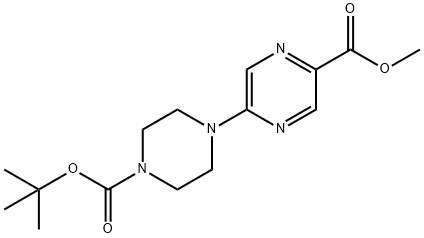
methyl 5-(4-(tert-butoxycarbonyl)piperazin-1-yl)pyrazine-2-carboxylate synthesis
- Product Name:methyl 5-(4-(tert-butoxycarbonyl)piperazin-1-yl)pyrazine-2-carboxylate
- CAS Number:1215626-40-6
- Molecular formula:C15H22N4O4
- Molecular Weight:322.36

57260-71-6
738 suppliers
$5.00/5g
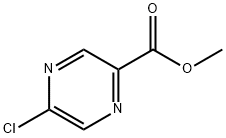
33332-25-1
246 suppliers
$8.00/1g

1215626-40-6
7 suppliers
inquiry
Yield:1215626-40-6 96%
Reaction Conditions:
with triethylamine in N,N-dimethyl-formamide at 80; for 3 h;
Steps:
84.1; 86.1 Step 1: Synthesis of methyl 5-[4-(tert-butoxycarbonyl)piperazin-1-yl]pyrazine- 2-carboxylate
methyl 5-chloropyrazine-2-carboxylate (532 mg, 3.08 mmol) and tert- butyl piperazine-1-carboxylate (631 mg, 3.39 mmol) were dissolved in DMF (4.85 mL). TEA (0.86 mL, 6.17 mmol) was added and the reaction mixture was heated at 80 °C for 3 hours. The reaction mixture was poured into water and filtered. The filtercake was dried under a stream of nitrogen while applying vacuum to yield the title compound (950 mg, 96%).
References:
WO2021/226262,2021,A1 Location in patent:Page/Page column 209; 212

57260-71-6
738 suppliers
$5.00/5g
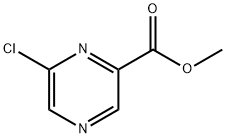
23611-75-8
127 suppliers
$9.00/250mg

1215626-40-6
7 suppliers
inquiry
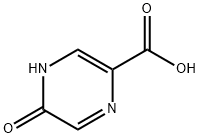
34604-60-9
207 suppliers
$15.00/1g

1215626-40-6
7 suppliers
inquiry