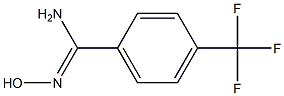
N'-hydroxy-4-(trifluoromethyl)benzenecarboximidamide synthesis
- Product Name:N'-hydroxy-4-(trifluoromethyl)benzenecarboximidamide
- CAS Number:1219624-50-6
- Molecular formula:C8H7F3N2O
- Molecular Weight:204.1492
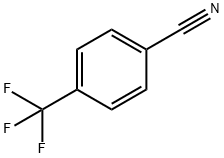
455-18-5
345 suppliers
$5.00/5g

1219624-50-6
2 suppliers
inquiry
Yield:1219624-50-6 95%
Reaction Conditions:
with hydroxylamine hydrochloride;sodium hydroxide in ethanol;water at 20; for 15 h;
Steps:
5 4.2.3 (Z)-N’-hydroxyimidamides (5a-e) [15]
General procedure: Aryl nitrile 4a-e (15.0mmol) was dissolved in ethanol (25mL) in a round-bottom two-necked balloon, under magnetic stirring, and hydroxylamine hydrochloride (2.085g, 30mmol) and aqueous NaOH (2 equiv, 10mL) were added to the mixture. The mixture was stirred at room temperature for 15-24h, monitoring the reaction by TLC. Then, the solvent was removed under rotary evaporation and water (20mL) was added to the crude mixture. The mixture was extracted with ethyl acetate (3×20mL) and the organic layer was dried over anhydrous MgSO4 and filtered. The solvent was removed by rotary evaporation and the product was recrystallized from chloroform - hexane.
References:
Mayer, Jo?o C.P.;Sauer, André C.;Iglesias, Bernardo A.;Acunha, Thiago V.;Back, Davi F.;Rodrigues, Oscar E.D.;Dornelles, Luciano [Journal of Organometallic Chemistry,2017,vol. 841,p. 1 - 11]