
5-chloro-6-iodo-2,3-dihydro-1H-1,3-benzodiazole-2-thione synthesis
- Product Name:5-chloro-6-iodo-2,3-dihydro-1H-1,3-benzodiazole-2-thione
- CAS Number:1219741-21-5
- Molecular formula:C7H4ClIN2S
- Molecular Weight:310.54

75-15-0
259 suppliers
$40.00/100g

1219741-20-4
20 suppliers
inquiry

1219741-21-5
42 suppliers
$30.00/100mg
Yield:1219741-21-5 80%
Reaction Conditions:
with potassium hydroxide in ethanol;water; for 3 h;Inert atmosphere;Reflux;
Steps:
From this, a 100-mL round-bottom flask purged and maintained with an inert atmosphere of nitrogen. A solution of 4-chloro-5-iodobenzene-1,2-diamine (2.69 g, 10.02 mmol, 1.00 equiv) in ethanol (50 mL), CS2 (6 g, 78.95 mmol, 8.00 equiv) and potassium hydroxide (1.68 g, 30.00 mmol, 3.00 equiv) was placed into the flask. The resulting solution was heated to reflux for 3 h. The resulting mixture was concentrated under vacuum. The resulting solution was diluted with 100 mL of H2O. The resulting solution was extracted with 3×100 mL of ethyl acetate and the organic layers combined. The resulting mixture was washed with 100 mL of brine. The mixture was dried over anhydrous sodium sulfate and concentrated under vacuum. This resulted in 2.5 g (80%) of 5-chloro-6-iodo-2,3-dihydro-1H-1,3-benzodiazole-2-thione as a brown solid.
References:
US2013/281392,2013,A1 Location in patent:Paragraph 0148

1219741-20-4
20 suppliers
inquiry

1219741-21-5
42 suppliers
$30.00/100mg
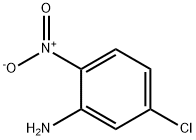
1635-61-6
300 suppliers
$13.00/25g

1219741-21-5
42 suppliers
$30.00/100mg

335349-57-0
61 suppliers
$16.00/250mg

1219741-21-5
42 suppliers
$30.00/100mg