
3-Chloro-2-fluoro-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine synthesis
- Product Name:3-Chloro-2-fluoro-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine
- CAS Number:1220219-73-7
- Molecular formula:C11H14BClFNO2
- Molecular Weight:257.5
38185-56-7
123 suppliers
$9.00/1g

73183-34-3
563 suppliers
$6.00/5g

1220219-73-7
36 suppliers
$68.00/250mg
Yield:1220219-73-7 72.1%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in 1,4-dioxane at 90; for 4 h;Inert atmosphere;
Steps:
91.1 STEP 1 (INTERMEDIATE B): 3-CHLORO-2-FLUORO-5-(4,4,5,5-TETRAMETHYL-l,3,2- DIOXABOROLAN-2-YL)PYRIDINE
STEP 1 (INTERMEDIATE B): 3-CHLORO-2-FLUORO-5-(4,4,5,5-TETRAMETHYL-l,3,2- DIOXABOROLAN-2-YL)PYRIDINE
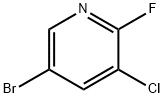
[00216] A mixture of 5 -bromo-3 -chloro-2-fluoropyridine (25. O g, 118.8 mmol, Ark Pharma), 4,4,4',4',5,5,5',5'-octamethyl-2,2'-bi(l,3,2-dioxaborolane) (36.2 g, 142.5 mmol) and potassium acetate (23.3 g, 237.6 mmol) in dioxane (250 mL) was degassed with nitrogen for 10 min. PdCl2(dppf)-CH2Cl2(4.85 g, 5.94 mmol) was added to the reaction mixture and and it was again degassed for 10 min. The reaction mixture was heated at 90 °C for 4 h. After completion, the reaction mixture was filtered through CELITE and the CELITE bed was washed with EtOAc (200 mL). The filtrate was concentrated under reduced pressure to afford the crude material which was purified by column chromatography (neutral alumina; elution: hexane) to afford Intermediate B as a white solid (22.0 g, 72.1%). MS (ESI, positive ion) m/z; No ionization.1H NMR (400 MHz, CDC13) δ 8.43 (s, 1H), 8.16 (dd, J= 9.6, 1.5 Hz, 1H), 1.34 (s, 12H).
References:
WO2015/51043,2015,A1 Location in patent:Paragraph 00216