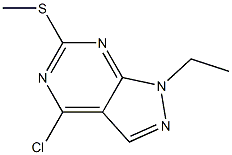
4-chloro-1-ethyl-6-(Methylthio)-1H-pyrazolo[3,4-d]pyriMidine synthesis
- Product Name:4-chloro-1-ethyl-6-(Methylthio)-1H-pyrazolo[3,4-d]pyriMidine
- CAS Number:1220517-81-6
- Molecular formula:C8H9ClN4S
- Molecular Weight:228.7019
Yield:1220517-81-6 77%
Reaction Conditions:
with sodium hydride in acetonitrile;mineral oil at 5 - 50; for 3 h;
Steps:
30 4-chloro-l-ethyl-6-(methylthio)-l/-/-pyrazolo[3,4-c/]pyrimidine (30j)
To a solution of (27i') (1.0 g, 5.0 mmol) in acetonitrile (10 mL) cooled at 5°C on an ice bath, were added NaH (144 mg, 6.0 mmol) and iodoethane (0.60 mL, 7.5 mmol). After 3 hours stirring at 50°C, acetonitrile was evaporated to dryness under vacuum and the residue was partitioned between water (50 mL) and dichloromethane (2 x 50 mL). The combined organic layers were dried and evaporated to dryness under vacuum. The residue was purified by silica gel column chromatography.Yield: 11%.Melting point: 92-93.5°C.H NMR (DMSO -d6) d 1.43 (t, J=7.2 Hz, 3H, NCH2C/ ,), 2.62 (s, 3H, S CH3), 4.42 (q, J=7.2 Hz, 2H, NCH2CH3), 8.34 (s, 1H, CH).13C NMR (DMSO -d6) d 13.9 (SCH3), 14.4 (NCH2CH3), 42.2 (NCH2CH3), 110.1 (C-3a), 132.3 (C-3), 153.0 (C-4/C-7a), 168.7 (C-6).
References:
WO2019/158655,2019,A1 Location in patent:Page/Page column 91; 92
6629-60-3
93 suppliers
$52.00/5g
33097-11-9
147 suppliers
$17.00/250mg
![4-chloro-1-ethyl-6-(Methylthio)-1H-pyrazolo[3,4-d]pyriMidine](/CAS/20130318/GIF/CB52599459.gif)
1220517-81-6
11 suppliers
inquiry

33097-11-9
147 suppliers
$17.00/250mg
![4-chloro-1-ethyl-6-(Methylthio)-1H-pyrazolo[3,4-d]pyriMidine](/CAS/20130318/GIF/CB52599459.gif)
1220517-81-6
11 suppliers
inquiry
1979-98-2
237 suppliers
$14.00/5g
![4-chloro-1-ethyl-6-(Methylthio)-1H-pyrazolo[3,4-d]pyriMidine](/CAS/20130318/GIF/CB52599459.gif)
1220517-81-6
11 suppliers
inquiry
![4-CHLORO-6-(METHYLTHIO)-1H-PYRAZOLO[3,4-D]PYRIMIDINE](/CAS/GIF/85426-79-5.gif)
