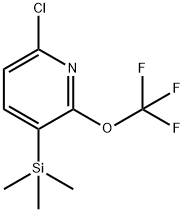
6-chloro-2-(trifluoroMethoxy)-3-(triMethylsilyl)pyridine synthesis
- Product Name:6-chloro-2-(trifluoroMethoxy)-3-(triMethylsilyl)pyridine
- CAS Number:1221172-02-6
- Molecular formula:C9H11ClF3NOSi
- Molecular Weight:269.72
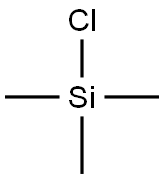
75-77-4
567 suppliers
$5.04/10
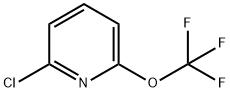
1221171-70-5
83 suppliers
$71.00/250mg

1221172-02-6
21 suppliers
inquiry
Yield:1221172-02-6 94%
Reaction Conditions:
Stage #1: 2-chloro-6-(trifluoromethoxy)pyridinewith n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -78; for 2.5 h;
Stage #2: chloro-trimethyl-silane in tetrahydrofuran;hexane at 20; for 1 h;
Steps:
6.c; 7.c
c) 6-chloro-2-(trifluoromethoxy)-3-(trimethylsilyl)pyridine (1-6); A LDA solution was prepared from n-BuLi 2.5M in hexane (22 mL; 56.80 mmol; 1.1 eq) and diisopropylamine (8 mL; 56.80 mmol; 1.1 eq) in THF (40 mL) at 0°C. To the LDA solution at -78 °C, a solution of 2-chloro-6-(trifluoromethoxy)pyridine (10.2 g; 51.64 mmol; 1 eq) in THF (40 mL) was added dropwise over 30 minutes. The solution was stirred at -78 °C for 2 hours, after what TMSCI (7.20 mL; 56.80 mmol; 1.1 eq) was added dropwise. The reaction mixture was allowed to warm up to room temperature and stirred 1 hour. Water (120 mL) was then added. The aqueous layer was extracted with diethylether (3 x 120 mL), and the combined organic layers were dried over sodium sulphate, filtered and concentrated under reduced pressure. The crude was purified on silica gel using petroleum ether (100 %) as an eluent to afford 6-chloro-2- (trifluoromethoxy)-3-(trimethylsilyl)pyridine in 94 % yield as a colorless oil.1H- MR (CDC13): δ (ppm) 0.33 (s, 9H); 7.21 (d, 1H); 7.77 (d, 1H).
References:
WO2012/93174,2012,A1 Location in patent:Page/Page column 38-39