
ethyl 2-broMo-6-forMyl-4H-thieno[3,2-b]pyrrole-5-carboxylate synthesis
- Product Name:ethyl 2-broMo-6-forMyl-4H-thieno[3,2-b]pyrrole-5-carboxylate
- CAS Number:1221186-54-4
- Molecular formula:C10H8BrNO3S
- Molecular Weight:302.14
![ethyl 2-bromo-4H-thieno[3,2-b]pyrrole-5-carboxylate](/CAS/GIF/238749-50-3.gif)
238749-50-3
74 suppliers
$61.00/250mg
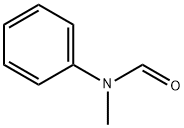
93-61-8
284 suppliers
$10.00/5G
![ethyl 2-broMo-6-forMyl-4H-thieno[3,2-b]pyrrole-5-carboxylate](/CAS/GIF/1221186-54-4.gif)
1221186-54-4
11 suppliers
inquiry
Yield:1221186-54-4 730 mg
Reaction Conditions:
with trichlorophosphate in 1,2-dichloro-ethane at 0 - 85;
Steps:
4.C Step C. Synthesis of ethyl 2-bromo-6-formyl-4H-thieno[3,2-b]pyrrole-5-carboxylate
To a mixture of ethyl 2-bromo-4H-thieno[3,2-b]pyrrole-5-carboxylate (720 mg, 2.6 mmol) in 1,2- dichloroethane (DCE) (20 mL) was added N-methyl-N-phenylformamide (530 mg, 3.9 mmol) and POCI3 (600 mg, 3.9 mmol) at 0 °C. The mixture was stirred at 85 °C overnight. The reaction mixture was poured into water, extracted with EtOAc. The combined organic layer was dried over anhy. Na2SO4 and concentrated. The residue was purified by silica gel chromatography to give ethyl 2-bromo-6-formyl-4H-thieno[3,2-b]pyrrole-5-carboxylate (730 mg). LC-MS (ESI): m/z 302 (M+H)+.
References:
WO2020/167976,2020,A1 Location in patent:Page/Page column 78-79

4701-17-1
299 suppliers
$6.00/1g
![ethyl 2-broMo-6-forMyl-4H-thieno[3,2-b]pyrrole-5-carboxylate](/CAS/GIF/1221186-54-4.gif)
1221186-54-4
11 suppliers
inquiry
1421933-23-4
0 suppliers
inquiry
![ethyl 2-broMo-6-forMyl-4H-thieno[3,2-b]pyrrole-5-carboxylate](/CAS/GIF/1221186-54-4.gif)
1221186-54-4
11 suppliers
inquiry