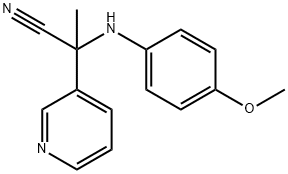
2-(4-methoxyphenylamino)-2-(pyridin-3-yl)propanenitrile synthesis
- Product Name:2-(4-methoxyphenylamino)-2-(pyridin-3-yl)propanenitrile
- CAS Number:1221756-04-2
- Molecular formula:C15H15N3O
- Molecular Weight:253.3

350-03-8
648 suppliers
$10.00/5g
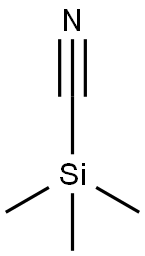
7677-24-9
379 suppliers
$19.00/5g
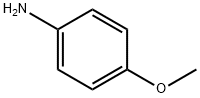
104-94-9
480 suppliers
$9.00/1g

1221756-04-2
1 suppliers
inquiry
Yield:1221756-04-2 87%
Reaction Conditions:
with triphenylphosphine;diethylazodicarboxylate in neat (no solvent) at 20; for 0.666667 h;
Steps:
Synthesis of alpha-aminonitriles from carbonyl compounds
General procedure: A mixture of aldehyde (1 mmol), amine (1 mmol), Mitsunobu’s reagent (1 mmol), and trimethylsilylcyanide (1.2 mmol) was added at room temperature and stirring was continued until the reaction was complete (monitored by TLC). After completion of the reaction, the reaction mixture was extracted with ethylacetate, dried over anhydrous Na2SO4, and concentrated. Purification of the crude product by chromatography on silica gel (60-120 mesh) with petroleum ether-EtOAc (5:1) as eluent gave the pure product
References:
Chaturvedi, Devdutt;Chaturvedi, Amit K.;Mishra, Nisha;Mishra, Virendra [Tetrahedron Letters,2012,vol. 53,# 40,p. 5398 - 5401]