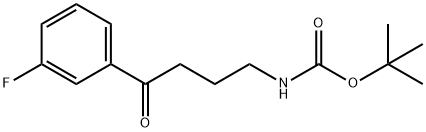
Carbamic acid, N-[4-(3-fluorophenyl)-4-oxobutyl]-, 1,1-dimethylethyl ester synthesis
- Product Name:Carbamic acid, N-[4-(3-fluorophenyl)-4-oxobutyl]-, 1,1-dimethylethyl ester
- CAS Number:1223405-24-0
- Molecular formula:C15H20FNO3
- Molecular Weight:281.32
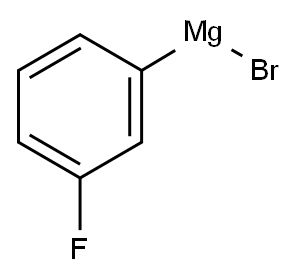
17318-03-5
87 suppliers
$60.00/100mg
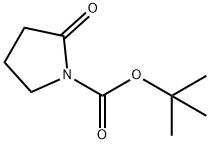
85909-08-6
241 suppliers
$6.00/1g
![Carbamic acid, N-[4-(3-fluorophenyl)-4-oxobutyl]-, 1,1-dimethylethyl ester](/CAS/20210111/GIF/1223405-24-0.gif)
1223405-24-0
1 suppliers
inquiry
Yield:1223405-24-0 72%
Reaction Conditions:
in tetrahydrofuran at -78 - 20; for 14 h;Inert atmosphere;Schlenk technique;
Steps:
tert-Butyl (4-Oxo-4-arylbutyl)carbamate (1): General Procedure
General procedure: The above prepared tert-Butyl-2-oxopyrrolidine-1-carboxylate (20 mmol) was dissolved in anhydrous THF (50 mL) in a three-necked, round-bottomed flask equipped with a mechanical stirrer and cooled to -78 °C under nitrogen. To the solution was added dropwise a solution of the RMgBr Grignard reagent (24 mmol). The reaction mixture was stirred at -78 °C for 2 h and then warmed slowly to room temperature for 12 more hours. Then it was quenched with 2 M HCl (30 mL) and extracted with CH2Cl2. The combined organic phases was washed with brine, dried over anhydrous Na2SO4, and concentrated under vacuum to yield the crude product, which was purified with column chromatography (EtOAc/Petroleum ether) to give the desired product.7,15
References:
Chang, Mingxin;Guo, Haodong;Huang, Haizhou;Zhang, Tao;Zhao, Wenlei;Zhou, Huan [Synthesis,2019,vol. 51,# 13,p. 2713 - 2719]
1073-06-9
441 suppliers
$6.00/10g

85909-08-6
241 suppliers
$6.00/1g
![Carbamic acid, N-[4-(3-fluorophenyl)-4-oxobutyl]-, 1,1-dimethylethyl ester](/CAS/20210111/GIF/1223405-24-0.gif)
1223405-24-0
1 suppliers
inquiry

24424-99-5
853 suppliers
$13.50/25G
![Carbamic acid, N-[4-(3-fluorophenyl)-4-oxobutyl]-, 1,1-dimethylethyl ester](/CAS/20210111/GIF/1223405-24-0.gif)
1223405-24-0
1 suppliers
inquiry