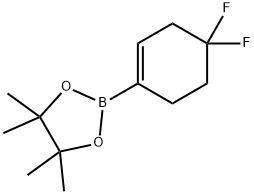
2-(4,4-Difluorocyclohex-1-en-1-yl)-4,4,5,5-tetraMethyl-1,3,2-dioxaborolane synthesis
- Product Name:2-(4,4-Difluorocyclohex-1-en-1-yl)-4,4,5,5-tetraMethyl-1,3,2-dioxaborolane
- CAS Number:1227068-84-9
- Molecular formula:C12H19BF2O2
- Molecular Weight:244.09
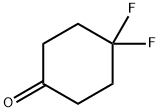
22515-18-0
235 suppliers
$5.00/100mg

73183-34-3
564 suppliers
$6.00/5g

1227068-84-9
77 suppliers
$21.00/100mg
Yield:1227068-84-9 49.5%
Reaction Conditions:
Stage #1:4,4-difluorocyclohexanone in tetrahydrofuran at -78; for 1 h;Alkaline conditions;
Stage #2: with N,N-phenylbistrifluoromethane-sulfonimide in tetrahydrofuran at -78 - 20; for 16 h;
Stage #3:bis(pinacol)diborane with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;potassium acetate in 1,4-dioxane at 100; for 16 h;
Steps:
23.1 Step 1
To a solution of 4,4-difluorocyclohexan-1-one (1.0 g, 7.5 mmol, Combi-Blocks) in THF (10 mL) was added LiHMDS (1.0 M in THF, 8.95 mL, 8.95 mmol) at -78°C. The reaction mixture was stirred for 1 h before 1,1,1-trifluoro-N-phenyl-N-((trifluoromethyl)sulfonyl)methanesulfonamide (2.93 g, 8.20 mmol) was added at -78°C and the resulting solution was slowly warmed to room temperature and stirred for 16 h. The reaction mixture was quenched with water and extracted with ethyl acetate. The organic extract was washed with brine, dried over Na2SO4, filtered, and concentrated. To a solution of the residue in 1,4-dioxane (20 mL) were added potassium acetate (1.46 g, 14.91 mmol), bispinacolato diboron (2.272 g, 8.95 mmol) and PdCl2(dppf) (0.546 g, 0.746 mmol). The reaction mixture was stirred at 100 °C for 16 h before it was diluted with water and was extracted with ethyl acetate. The organic extract was washed with brine, dried over Na2SO4, filtered, concentrated and purified by flash column chromatography, eluting with a gradient of 10 % to 20 % ethyl acetate in petroleum ether to provide 2- (4,4-difluorocyclohex-1-en-1-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (900 mg, 3.69 mmol, 49.5 % yield) as yellow oil.1H NMR (400 MHz, Chloroform-d): d ppm 6.41 (t, J=3.7 Hz, 1 H), 2.53- 2.68 (m, 2 H), 2.43 (t, J=6.7 Hz, 2 H), 1.91- 2.06 (m, 2 H), 1.29 (s, 12 H).
References:
AMGEN INC.;TAMAYO, Nuria A.;BANERJEE, Abhisek;BROWN, James Alexander;FROHN, Michael J.;CHEN, Jian Jeffrey;LI, Kexue;LIU, Qingyian;LOW, Jonathan Dante;MA, Vu;PETTUS, Liping H.;WALTON, Mary Catherine;MINATTI, Ana Elena;BOURBEAU, Matthew Paul;JIA, Lei;NGUYEN, Thomas T.;NISHIMURA, Nobuko;XUE, Qiufen May;ALLEN, John Gordon WO2020/132651, 2020, A1 Location in patent:Paragraph 0319
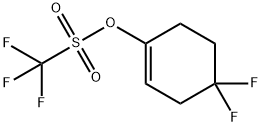
1227068-83-8
3 suppliers
inquiry

73183-34-3
564 suppliers
$6.00/5g

1227068-84-9
77 suppliers
$21.00/100mg

22515-18-0
235 suppliers
$5.00/100mg

1227068-84-9
77 suppliers
$21.00/100mg