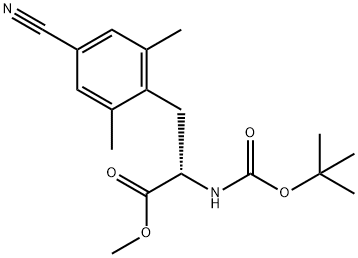
(S)-methyl 2-((tert-butoxycarbonyl)amino)-3-(4-cyano-2,6-dimethylphenyl)propanoate synthesis
- Product Name:(S)-methyl 2-((tert-butoxycarbonyl)amino)-3-(4-cyano-2,6-dimethylphenyl)propanoate
- CAS Number:1227311-10-5
- Molecular formula:C18H24N2O4
- Molecular Weight:332.39
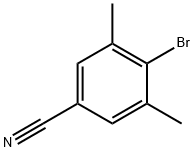
75344-77-3
158 suppliers
$6.00/100mg
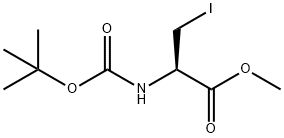
93267-04-0
247 suppliers
$5.00/250mg

1227311-10-5
13 suppliers
inquiry
Yield:1227311-10-5 50%
Reaction Conditions:
Stage #1: N-(tert-butoxycarbonyl)-L-3-iodoalanine methyl esterwith iodine;zinc in N,N-dimethyl-formamide at 20; for 0.666667 h;Inert atmosphere;
Stage #2: 4-bromo-3,5-dimethyl benzonitrilewith dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane;tris-(dibenzylideneacetone)dipalladium(0) in N,N-dimethyl-formamide at 50;Temperature;
Steps:
A5.1; B8.1 Step 1: Synthesis of methyl OSy2-(Yfer/-butoxycarbonvOamino)-3-(4-cvano- 2.6-dim ethyl phenyl ipropanoate
To a stirred suspension of zinc powder (2.1 g, 32.3 mmol) in anhydrous N,N-d m ethyl form a ide (10 mL) under nitrogen was added iodine (170 mg). After 5min, a solution of methyl (i?)-2-((/er/-butoxycarbonyl)amino)-3-iodopropanoate (4.2 g, 12.8 mmol) in A( A'-di m ethyl form a i de (10 mL) was added slowly over lOmin. The mixture was stirred at rt for 30min. A suspension of 4-bromo-3,5-dimethylbenzonitrile (2.5 g, 12 mmol), Pd2(dba)3 (205 mg, 0.22 mmol), and 2-dicyclohexylphosphino-2',6'- dimethoxybiphenyl (Sphos, 210 mg, 0.51 mmol) in A( A-di m eth yl form am i de (10 mL) was added into the zinc reagent mixture. The reaction mixture was heated at 50°C and stirred overnight. After cooling, the reaction mixture was quenched with water, diluted with EtOAc and filtered through Celite. The filter cake was washed with EtOAc and the filtrate was washed with brine, dried over anhydrous sodium sulfate, and concentrated. The residue was purified by flash column chromatography over silica gel (0-20% EtOAc/CH2Cl2) to afford the desired product as a white solid (2.0 g, 50%). LC-MS: 355.2 [M+Na]+; 1H NMR (400MHz, CDCl3) d 7.30 (s, 2H), 5.13 (d, 1H, J = 8.8 Hz), 4.56 (q, 1H, J = 8.0 Hz), 3.67 (s, 3H), 3.15-3.00 (m, 2H), 2.38 (s, 6H), 1.35 (s, 9H).
References:
WO2019/195634,2019,A1 Location in patent:Page/Page column 87-88; 151-152
4326-36-7
354 suppliers
$6.00/5g

1227311-10-5
13 suppliers
inquiry
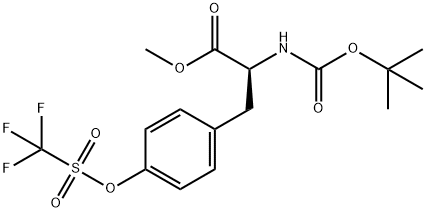
112766-18-4
29 suppliers
inquiry

1227311-10-5
13 suppliers
inquiry
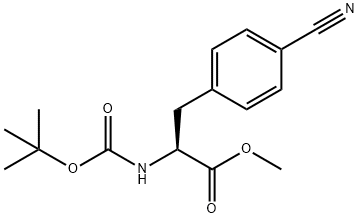
298693-79-5
7 suppliers
inquiry

1227311-10-5
13 suppliers
inquiry
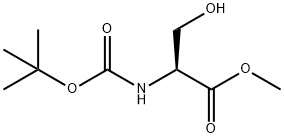
2766-43-0
298 suppliers
$10.00/1g

1227311-10-5
13 suppliers
inquiry