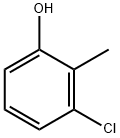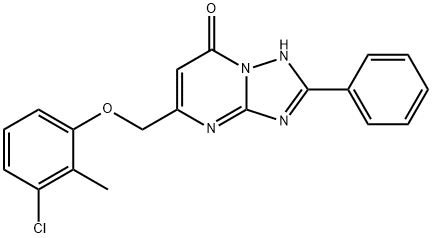
[1,2,4]Triazolo[1,5-a]pyrimidin-7(1H)-one, 5-[(3-chloro-2-methylphenoxy)methyl]-2-phenyl- synthesis
- Product Name:[1,2,4]Triazolo[1,5-a]pyrimidin-7(1H)-one, 5-[(3-chloro-2-methylphenoxy)methyl]-2-phenyl-
- CAS Number:1227413-92-4
- Molecular formula:C19H15ClN4O2
- Molecular Weight:366.8
Yield:1227413-92-4 31%
Reaction Conditions:
Stage #1: 3-chloro-o-cresolwith sodium hydride in ISOPROPYLAMIDE;mineral oil at 20; for 0.25 h;
Stage #2: 5-(chloromethyl)-2-phenyl-[1,2,4]triazolo[1,5-a]pyrimidin-7(4H)-one in ISOPROPYLAMIDE;mineral oil at 210; for 0.5 h;Microwave irradiation;
Steps:
1.B
Part B; A reaction mixture of 3-chloro-2-methylphenol (356.5 mg, 2.5 mmol) and sodium hydride (60% in oil, 120 mg, 3.0 mmol) in DMA (3 mL) was stirred at room temperature for 15 minutes. To the resultant clear solution was added compound 1-1 (390 mg, 1.5 mmol). The reaction mixture was stirred in microwave at 210 0C for 30 minutes. Recrystallization from a mixture of DMSO/ACN (4/1) gave compound 1-2 in a white solid (170.8 mg, 31% yield). 1H NMR (400 MHz, CD3OD) δ 8.25-8.15 (m, 2H), 7.55-7.45 (m, 3H), 7.20-7.12 (m, 1 H), 7.09-7.03 (m, 1H), 7.01-6.95 (m, 1H), 6.24 (s, 1 H), 5.15 (s, 2H), 2.37 (m, 3H).
References:
WO2010/56631,2010,A1 Location in patent:Page/Page column 84
![5-(chloromethyl)-2-phenyl-[1,2,4]triazolo[1,5-a]pyrimidin-7(1H)-one](/CAS/20180703/GIF/1227417-79-9.gif)