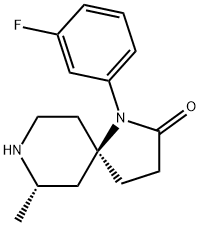
(5R,7S)-1-(3-fluorophenyl)-7-Methyl-1,8-diazaspiro[4.5]decan-2-one synthesis
- Product Name:(5R,7S)-1-(3-fluorophenyl)-7-Methyl-1,8-diazaspiro[4.5]decan-2-one
- CAS Number:1227685-16-6
- Molecular formula:C15H19FN2O
- Molecular Weight:262.32
![benzyl (5R,7S)-1-(3-fluorophenyl)-7-methyl-2-oxo-1,8-diazaspiro[4.5]dec-3-ene-8-carboxylate](/CAS/20180529/GIF/1227685-14-4.gif)
1227685-14-4
2 suppliers
inquiry
![(5R,7S)-1-(3-fluorophenyl)-7-Methyl-1,8-diazaspiro[4.5]decan-2-one](/CAS/20150408/GIF/1227685-16-6.gif)
1227685-16-6
6 suppliers
inquiry
Yield:1227685-16-6 71%
Reaction Conditions:
with hydrogen;Pearlman's catalist in methanol; under 2327.23 Torr; for 18 h;
Steps:
4
Preparation 4; (SR 7SV- 1 -(3-fluorophen yl)-7-methyl-1 ,8-diazasρirof4.5]decan~2-one (P4)C17 P4Synthesis of P4. Compound C17 (150 mg, 0.38 mmol) and palladium hydroxide (20% by weight on carbon, 26.7 mg, 0.038 mmol) were combined in methanol (4.75 ml) and hydrogenated for 18 hours under 45 psi of hydrogen. The reaction mixture was filtered through an Acrodisd2' syringe filter and the filtrate was concentrated in vacuo to afford P4 as an oil. Yield: 70 mg, 0.27 mmol, 71%, LCMS m/z 263.5 (M+1 ). 5H NMR (400 MHz, CDCI3) 6 1.08 (d, J=6.4 Hz, 3H), 1.59 {dd, J-U.2, 9.2 Hz, 1H), 1.86 (ddd, J=U. Z. 10.2, 4.5 Hz5 1H)5 1.98-2.14 (m, 4H), 2.50- 2.58 (m, 3H)1 2.76 (m, 1 H). 2,88 {, J=13.0, 4,9. 4,9 Hz, 1H), 6.84 (ddd, J-9,3, 2.2, 2.2 Hz, 1 H), 8.90 Cm, 1 H), 7.06 (dddd, >8.3, 8.3, 2.4, 0.9 Hz, 1H), 7.35 (ddd, J=8.2, 8.2, 6,4 Hz, 1H).
References:
WO2010/58333,2010,A1 Location in patent:Page/Page column 46