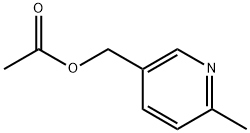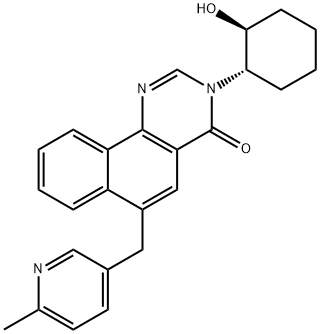
3-[(1S,2S)-2-Hydroxycyclohexyl]-6-[(6-methyl-3-pyridinyl)methyl]benzo[h]quinazolin-4(3H)-one synthesis
- Product Name:3-[(1S,2S)-2-Hydroxycyclohexyl]-6-[(6-methyl-3-pyridinyl)methyl]benzo[h]quinazolin-4(3H)-one
- CAS Number:1227923-29-6
- Molecular formula:C25H25N3O2
- Molecular Weight:399.48
Yield: 92%
Reaction Conditions:
with palladium diacetate;potassium carbonate;ruphos in water;isopropyl alcohol at 82; for 1 h;
Steps:
3.2 Step 2
Pinacol boronate 9A (30.0 g), palladium acetate (80.8 mg) and Ru-Phos (331 mg) were charged to areaction flask followed by a solution of commercially available pyridine acetate 1 OA (14.2 g) in IPA(47.1 mL). IPA (300.6 mL), water (30 mL), and potassium carbonate (29.6 g) were then added. The reaction mixture was sparged sub-surface with nitrogen for 5 minutes. The reaction was heated to 82 °C for 1 h. The reaction was cooled to 21 °C then diluted with water (75 mL) and MTBE (75.5 mL). The layers were separated and the organic layer was washed successively with 25 wt% NaC1 aqueous solution(100 g) and 25 weight (wt)% ammonium chloride aqueous solution (100 g). The organic layer was thendiluted with MTBE (75.5 mL) and water (75 mL). The layers were separated and the organic layer wasfiltered and concentrated to 37.5 mL, and MP-TMT resin (4.5 g), IPA (25.4 mL), and MTBE (5.4 mL)were added to the filtrate. The mixture was aged for 10 h, filtered and the product was crystallized from amixture of toluene (90 mL) and heptane (440 mL) to give 26.2 g in 92% isolated yield, >99 wt%, >99LCAP after filtration and washing with a mixture of toluene (20 mL) and heptane (50 mL).
References:
MERCK SHARP & DOHME CORP.;MERCK SHARP & DOHME LIMITED;CHEN, Qinghao;SCOTT, Jeremy;KRSKA, Shane, W.;BAXTER, Carl;STEWART, Gavin William;TAN, Lushi;GIBB, Andrew;MALIGRES, Peter, E. WO2017/58691, 2017, A1 Location in patent:Page/Page column 17
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![3-[(1S,2S)-2-Hydroxycyclohexyl]-6-[(6-methyl-3-pyridinyl)methyl]benzo[h]quinazolin-4(3H)-one](/CAS/20150408/GIF/1227923-29-6.gif)
1227923-29-6
44 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
5158-46-3
26 suppliers
$45.00/250mg
![3-[(1S,2S)-2-Hydroxycyclohexyl]-6-[(6-methyl-3-pyridinyl)methyl]benzo[h]quinazolin-4(3H)-one](/CAS/20150408/GIF/1227923-29-6.gif)
1227923-29-6
44 suppliers
inquiry
1227924-42-6
11 suppliers
inquiry
![3-[(1S,2S)-2-Hydroxycyclohexyl]-6-[(6-methyl-3-pyridinyl)methyl]benzo[h]quinazolin-4(3H)-one](/CAS/20150408/GIF/1227923-29-6.gif)
1227923-29-6
44 suppliers
inquiry

1227924-43-7
2 suppliers
inquiry
![3-[(1S,2S)-2-Hydroxycyclohexyl]-6-[(6-methyl-3-pyridinyl)methyl]benzo[h]quinazolin-4(3H)-one](/CAS/20150408/GIF/1227923-29-6.gif)
1227923-29-6
44 suppliers
inquiry