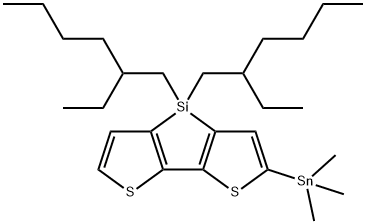
4H-Silolo[3,2-b:4,5-b']dithiophene, 4,4-bis(2-ethylhexyl)-2-(trimethylstannyl)- synthesis
- Product Name:4H-Silolo[3,2-b:4,5-b']dithiophene, 4,4-bis(2-ethylhexyl)-2-(trimethylstannyl)-
- CAS Number:1228237-08-8
- Molecular formula:C27H46S2SiSn
- Molecular Weight:581.58
![4,4'-Bis(2-ethyl-hexyl)-5,5'-bis(triMethyltin)-dithieno[3,2-b:2,3-d]silole](/CAS/20200611/GIF/1089687-06-8.gif)
1089687-06-8
28 suppliers
inquiry
![4H-Silolo[3,2-b:4,5-b']dithiophene, 4,4-bis(2-ethylhexyl)-2-(trimethylstannyl)-](/CAS/20210111/GIF/1228237-08-8.gif)
1228237-08-8
3 suppliers
inquiry
Yield:1228237-08-8 94%
Reaction Conditions:
with potassium carbonate;triethylamine in hexane;Reagent/catalyst;
Steps:
A2 Comparative Example A1
Silica gel (Kanto Chemical’s product name, Silica Gel 60N, spherical neutral, for column chromatography(particle size 63 to 210 mm, pH 7.0 6 0.5), 20 g) and anhydrous potassium carbonate (Aldrich’s Catalog No. 347825,powder, 2.0 g) were suspended in hexane (50 mL), and charged in a column (inner diameter 15 mm, length 5 cm).(Hereinafter the column material is referred to as silica gel/potassium carbonate.) The compound E2 (1.0 g) obtainedin Synthesis Example 1 was dissolved in hexane (5.0 mL), and charged in the column. Using hexane as a developingsolvent, the solution having passed through the column was collected. The solvent was evaporated away from thesolution under reduced pressure to give an oily compound (0.96 g, yield 96%). The obtained compound was analyzed through the above-mentioned proton NMR, in which neither the disubstitutedform (compound E2: Ar(2)) nor the mono-substituted form (compound S1: Ar(1)) derived from the compoundE2 by removing one trimethylstannyl group therefrom was present but only the unsubstituted form (compound S2: Ar(0))derived from the compound E2 by removing two trimethylstannyl groups therefrom was present. The proportion (by mol) of the mono-substituted form (Ar(1)) and the unsubstituted form (Ar(0)) in the compoundafter charging in the column was 100%, and the recovery efficiency of the di-substituted form was 0%. The recoveryefficiency (%) of the di-substituted form means di-substituted form (g) in the compound after charging in the column/disubstitutedform (g) before charging in the column 3 100. This is the same as in Comparative Example 1, except that in the process of Comparative Example A1, silicagel (Kanto Chemical’s product name, Silica Gel 60N, spherical neutral, for column chromatography (particle size 63 to210 mm, pH 7.0 6 0.5), 20 g) was used as the column material in place of silica gel/potassium carbonate, and thathexane containing 10% by weight of triethylamine was used as the developing solvent. As a result of the treatment here,an oily compound (0.94 g, yield 94%) was obtained. The obtained compound was analyzed through proton NMR, whichwas confirmed as a mixture of the compound E2 (di-substituted form (Ar(2)) and the compound S1 (mono-substitutedform (Ar(1)) derived from the compound E2 by removing one trimethylstannyl group therefrom. The ratio of the compoundE2 to the compound S 1 was 1/3 as the integration ratio of the chemical shift. In the compound after charging in the column, the ratio (by mol) of the mono-substituted form (Ar(1)) to theunsubstituted form (Ar(0)) was 75%, and the recovery efficiency of the di-substituted form was 24%.
References:
EP2774931,2014,A1 Location in patent:Paragraph 0239; 0240; 0241; 0242; 0243
![4,4-di-2-ethylhexyl-dithieno[3,2-b:2',3'-d]silole](/CAS/GIF/1207627-85-7.gif)
1207627-85-7
52 suppliers
inquiry

1066-45-1
146 suppliers
$26.03/2g
![4H-Silolo[3,2-b:4,5-b']dithiophene, 4,4-bis(2-ethylhexyl)-2-(trimethylstannyl)-](/CAS/20210111/GIF/1228237-08-8.gif)
1228237-08-8
3 suppliers
inquiry
![4,4'-Bis(2-ethyl-hexyl)-5,5'-bis(triMethyltin)-dithieno[3,2-b:2,3-d]silole](/CAS/20200611/GIF/1089687-06-8.gif)
1089687-06-8
28 suppliers
inquiry
![4,4-di-2-ethylhexyl-dithieno[3,2-b:2',3'-d]silole](/CAS/GIF/1207627-85-7.gif)
1207627-85-7
52 suppliers
inquiry
![4H-Silolo[3,2-b:4,5-b']dithiophene, 4,4-bis(2-ethylhexyl)-2-(trimethylstannyl)-](/CAS/20210111/GIF/1228237-08-8.gif)
1228237-08-8
3 suppliers
inquiry
![4,4-di-2-ethylhexyl-dithieno[3,2-b:2',3'-d]silole](/CAS/GIF/1207627-85-7.gif)
1207627-85-7
52 suppliers
inquiry
![4H-Silolo[3,2-b:4,5-b']dithiophene, 4,4-bis(2-ethylhexyl)-2-(trimethylstannyl)-](/CAS/20210111/GIF/1228237-08-8.gif)
1228237-08-8
3 suppliers
inquiry