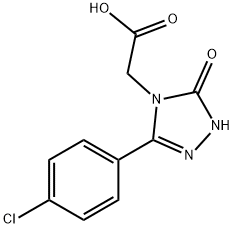
4H-1,2,4-Triazole-4-acetic acid, 3-(4-chlorophenyl)-1,5-dihydro-5-oxo- synthesis
- Product Name:4H-1,2,4-Triazole-4-acetic acid, 3-(4-chlorophenyl)-1,5-dihydro-5-oxo-
- CAS Number:1228650-22-3
- Molecular formula:C10H8ClN3O3
- Molecular Weight:253.64
![Benzoic acid, 4-chloro-, 2-[[(2-ethoxy-2-oxoethyl)amino]carbonyl]hydrazide](/CAS/20210305/GIF/1228650-21-2.gif)
1228650-21-2
0 suppliers
inquiry

1228650-22-3
2 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: ethyl N-({2-[(4-chlorophenyl)carbonyl]hydrazinyl}carbonyl)glycinatewith water;sodium hydroxideReflux;
Stage #2: with hydrogenchloride in water at 20; pH=1;
Steps:
2A
Example 2A[3-(4-Chlorophenyl)-5-oxo-1,5-dihydro-4H-1,2,4-triazol-4-yl]acetic acid Of the compound from Example 1A, 21.43 g (67.93 mmol) were admixed with 91 ml of a 3N aqueous sodium hydroxide solution and heated at reflux overnight. After cooling to RT, the mixture was adjusted to a pH of 1 by slow addition of approximately 20% strength hydrochloric acid. The precipitated solid was isolated by filtration, washed with water and dried at 60° C. under reduced pressure. Yield: 17.55 g (90% of theory, approximately 88% purity).LC/MS [Method 1]: Rt=0.94 min; m/z=254 (M+H)+ 1H NMR (DMSO-d6, 400 MHz): δ=13.25 (br.s, 1H), 12.09 (s, 1H), 7.65-7.56 (m, 4H), 4.45 (s, 2H).
References:
US2010/261771,2010,A1 Location in patent:Page/Page column 23
536-40-3
188 suppliers
$5.00/1g

1228650-22-3
2 suppliers
inquiry