Methanesulfonic acid, 1,1,1-trifluoro-, 4-phenyl-1-cyclohexen-1-yl ester synthesis
- Product Name:Methanesulfonic acid, 1,1,1-trifluoro-, 4-phenyl-1-cyclohexen-1-yl ester
- CAS Number:122948-47-4
- Molecular formula:C13H13F3O3S
- Molecular Weight:306.3
Yield:122948-47-4 89%
Reaction Conditions:
with 2,6-di-tert-butyl-4-methylpyridine in dichloromethane at 40; for 1 h;
Steps:
4
0.579 ml of trifluoromethanesulphonic anhydride (1.2 equivalents) is added dropwise to a solution of 0.5 g of 4-phenylcyclohexanone and 0.648 g of 2,6-di(tert-butyl)-4-methylpyridine (1.1 equivalents) in 8.0 ml of anhydrous dichloromethane. The reaction medium is subsequently stirred for 1 hour at 40° C., diluted with 25 ml of dichloromethane, washed with water (2×15 ml), dried over sodium sulphate, filtered and then concentrated under reduced pressure. Chromatography of the residue on silica gel (cyclohexane/ethyl acetate: 80/20) makes it possible to isolate 0.788 g of the expected product. Yield: 89% 1H NMR (CDCl3) δ (ppm): 1.92 (m, 1H), 2.05 (m, 1H), 2.35 (m, 4H), 2.80 (m, 1H), 5.80 (m, 1H), 7.15 (m, 3H), 7.22 (m, 2H)
References:
US2004/72871,2004,A1 Location in patent:Page/Page column 16
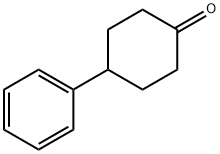
4894-75-1
168 suppliers
$6.00/5g

122948-47-4
1 suppliers
inquiry
![1,4-dioxaspiro[4.5]dec-7-en-8-yl trifluoromethanesulfonate](/CAS/GIF/170011-47-9.gif)
170011-47-9
43 suppliers
$330.72/250mg

122948-47-4
1 suppliers
inquiry