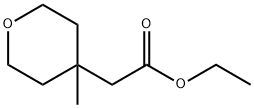
2H-Pyran-4-acetic acid, tetrahydro-4-methyl-, ethyl ester synthesis
- Product Name:2H-Pyran-4-acetic acid, tetrahydro-4-methyl-, ethyl ester
- CAS Number:1232672-20-6
- Molecular formula:C10H18O3
- Molecular Weight:186.25
130312-00-4
80 suppliers
$40.00/250mg
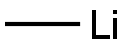
917-54-4
173 suppliers
$52.10/25ml

1232672-20-6
1 suppliers
inquiry
Yield:1232672-20-6 86%
Reaction Conditions:
Stage #1: methyllithiumwith copper(l) iodide in diethyl ether at 0; for 0.166667 h;Inert atmosphere;
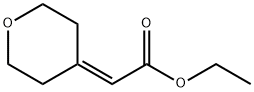
Stage #2: ethyl tetrahydro-4H-pyran-4-ylideneacetatewith chloro-trimethyl-silane in dichloromethane at -78 - 0; for 2.5 h;
Steps:
399.2 Step 2. Ethyl 2-(4-methyloxan-4-yl )acetate.
Step 2. Ethyl 2-(4-methyloxan-4-yl )acetate. To a stirred solution of Cul (2.9 g, 1 .23 mmol, 3.24 equiv) i n ether (50 mL) maintained under n itrogen at 0"C was added a 1 .6 M solution of mcthyllithium (20 mL, 32mmol, 6 8 equiv) in ether dropwise. The resulting solution was stirred at 0"C for 1 0 min and the ether solvent was removed from the reaction vessel under vacuum at 0"C. DC (50 mL) was then added to the res idue and the reaction was cooled to -78°C. Chlorotrimethylsilane ( 1 .9 g, 1 7.6 mmol, 3.7 equiv) was then added dropwise followed by a solution of ethyl 2- (oxan-4-ylidene)acetate (800 mg, 4.70 mmol, 1 00 equiv) in DCM (20 mL) to the reaction mixture at -78"C. The resulting solution was stirred for another 30 min at - 78°C and then at 0"C for 2 h. The reaction quenched by the addition of 50 mL of water. The organic layer was dried over anhydrous sodium sulfate and concentrated under vacuum to give 750 mg (86%) of ethyl 2-(4-methyloxan-4-yl)acetate as a colorless oi l
References:
WO2013/127269,2013,A1 Location in patent:Page/Page column 181

130312-00-4
80 suppliers
$40.00/250mg
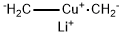
15681-48-8
8 suppliers
inquiry

1232672-20-6
1 suppliers
inquiry
29943-42-8
421 suppliers
$5.00/1g

1232672-20-6
1 suppliers
inquiry