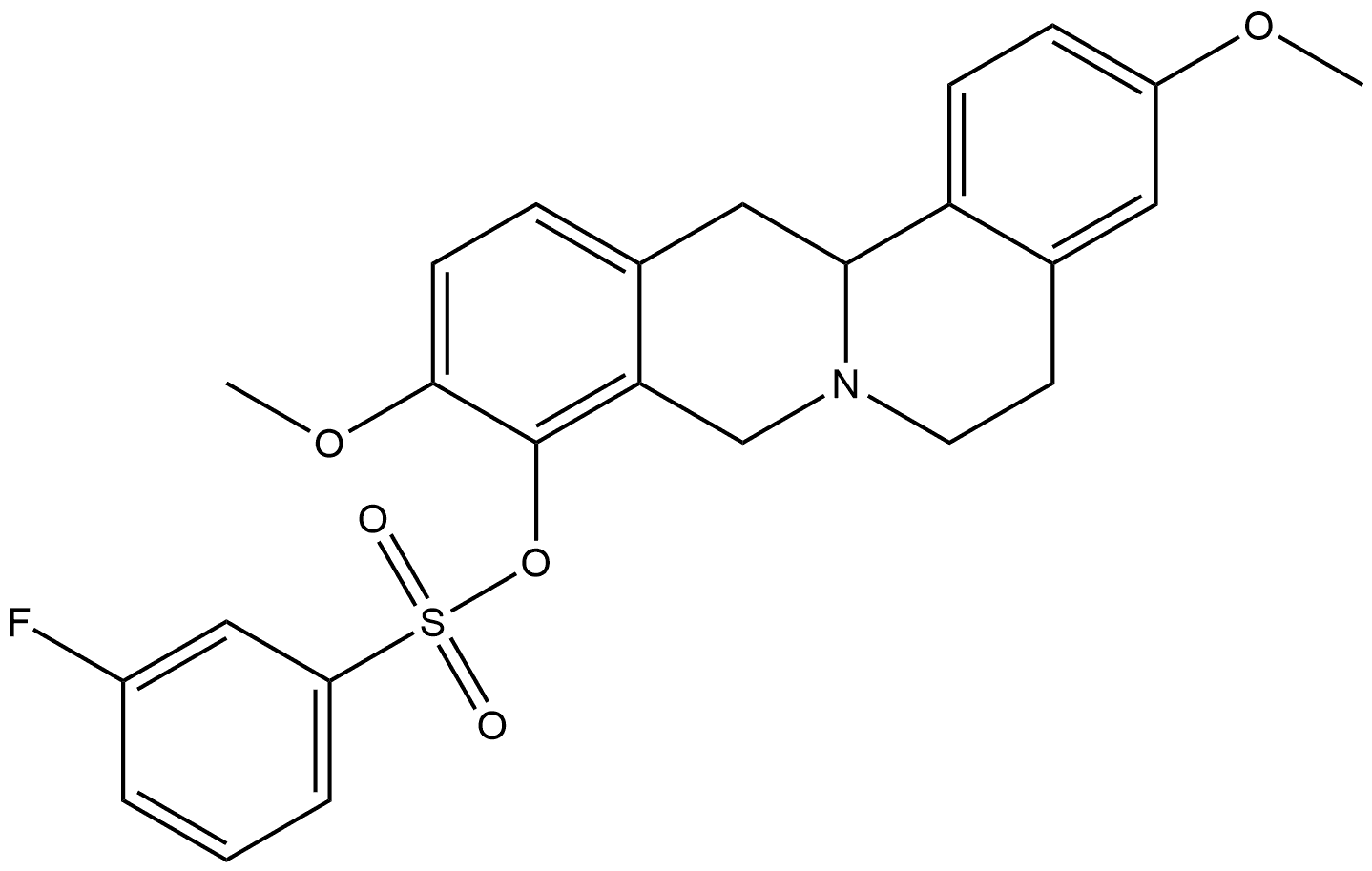
3,10-dimethoxy-5,8,13,13a-tetrahydro-6H-isoquinolino[3,2-a]isoquinolin-9-yl 3-fluorobenzenesulfonate synthesis
- Product Name:3,10-dimethoxy-5,8,13,13a-tetrahydro-6H-isoquinolino[3,2-a]isoquinolin-9-yl 3-fluorobenzenesulfonate
- CAS Number:1233354-53-4
- Molecular formula:C25H24FNO5S
- Molecular Weight:469.53
![Benzenesulfonic acid, 3-fluoro-, 2-(hydroxymethyl)-6-methoxy-3-[2-[[2-(3-methoxyphenyl)ethyl]amino]-2-oxoethyl]phenyl ester](/CAS/20210305/GIF/1233354-62-5.gif)
1233354-62-5
0 suppliers
inquiry
![3,10-dimethoxy-5,8,13,13a-tetrahydro-6H-isoquinolino[3,2-a]isoquinolin-9-yl 3-fluorobenzenesulfonate](/CAS/20210305/GIF/1233354-53-4.gif)
1233354-53-4
1 suppliers
inquiry
Yield:1233354-53-4 7.2%
Reaction Conditions:
Stage #1: 2-(hydroxymethyl)-6-methoxy-3-({[2-(3-methoxyphenyl)ethyl]carbamoyl}methyl)phenyl-3-fluorobenzene-1-sulfonatewith trichlorophosphate in toluene; for 2 h;Reflux;
Stage #2: with sodium tetrahydroborate in methanol at 20; for 0.5 h;Cooling with ice;
Steps:
Preparation of the Reference compound (C-1) Scheme:
To a 25mL round bottom flask was added 2-hydroxymethyl-6-methoxy-3-(2-((3-methoxy-phenylethyl)amino)-2-oxoethyl) phenyl-3-fluorosulfonate (45mg, 0.089mmol), toluene (5mL), was added with stirring phosphorus oxychloride (300μL), the reaction refluxed for 2h, the reaction solution was spin dry, the residue was cooled in an ice bath, was added a saturated aqueous sodium bicarbonate solution, extracted with ethyl acetate, washed with water, dried over anhydrous sodium sulfate, the organic phase spin dry, the residue was washed with methanol (2mL) was dissolved, placed in an ice bath was added portionwise sodium borohydride (14mg, 0.357mmol), the addition was completed, stirring at room temperature 30min, the reaction solution was spin-dried, the residue was added water and ethyl acetate, extracted layers, the organic phase was collected, dried over anhydrous sulfate sodium sulfate, and spin dry, the residue was purified by preparative thin-layer chromatography (dichloromethane: methanol 50: 1) gave an off-white solid (3mg, 7.2% yield).
References:
CN105481849,2016,A Location in patent:Paragraph 0460; 0461; 0473; 0474
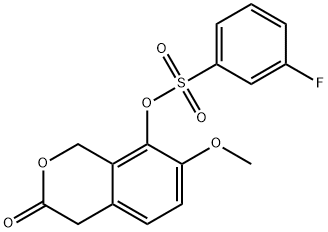
1233354-60-3
0 suppliers
inquiry
![3,10-dimethoxy-5,8,13,13a-tetrahydro-6H-isoquinolino[3,2-a]isoquinolin-9-yl 3-fluorobenzenesulfonate](/CAS/20210305/GIF/1233354-53-4.gif)
1233354-53-4
1 suppliers
inquiry
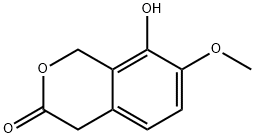
56201-87-7
7 suppliers
inquiry
![3,10-dimethoxy-5,8,13,13a-tetrahydro-6H-isoquinolino[3,2-a]isoquinolin-9-yl 3-fluorobenzenesulfonate](/CAS/20210305/GIF/1233354-53-4.gif)
1233354-53-4
1 suppliers
inquiry
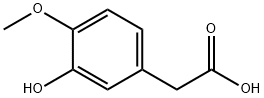
1131-94-8
176 suppliers
$9.00/250mg
![3,10-dimethoxy-5,8,13,13a-tetrahydro-6H-isoquinolino[3,2-a]isoquinolin-9-yl 3-fluorobenzenesulfonate](/CAS/20210305/GIF/1233354-53-4.gif)
1233354-53-4
1 suppliers
inquiry
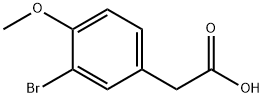
774-81-2
116 suppliers
$23.00/5g
![3,10-dimethoxy-5,8,13,13a-tetrahydro-6H-isoquinolino[3,2-a]isoquinolin-9-yl 3-fluorobenzenesulfonate](/CAS/20210305/GIF/1233354-53-4.gif)
1233354-53-4
1 suppliers
inquiry