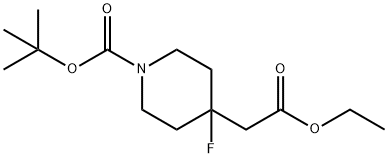
4-Piperidineacetic acid, 1-[(1,1-diMethylethoxy)carbonyl]-4-fluoro-, ethyl ester synthesis
- Product Name:4-Piperidineacetic acid, 1-[(1,1-diMethylethoxy)carbonyl]-4-fluoro-, ethyl ester
- CAS Number:1235842-48-4
- Molecular formula:C14H24FNO4
- Molecular Weight:289.34
![tert-Butyl 4-[(ethoxycarbonyl)methyl]-4-hydroxypiperidine-1-carboxylate](/CAS/GIF/401811-97-0.gif)
401811-97-0
17 suppliers
inquiry
![4-Piperidineacetic acid, 1-[(1,1-diMethylethoxy)carbonyl]-4-fluoro-, ethyl ester](/CAS/GIF/1235842-48-4.gif)
1235842-48-4
23 suppliers
inquiry
Yield:1235842-48-4 11%
Reaction Conditions:
with diethylamino-sulfur trifluoride in dichloromethane at -78; for 2 h;
Steps:
2 Step 2
To a solution of tert-butyl 4-(2-ethoxy-2-oxo-ethyl)-4-hydroxy-piperidine-l-carboxylate (5 g, 17.4 mmol, 1 eq) in dichloromethane (50 mL) was added diethylaminosulfur trifluoride (4.21 g, 26.1 mmol, 1.5 eq) at -78 °C. The mixture was stirred at -78 °C for 2 hr. The mixture was then poured into aqueous sodium bicarbonate (200 mL), extracted with ethyl acetate (200 mL + 100 mL), organic layer was dried over sodium sulfate, and solvent was removed under vacuum. The oily residue was taken into ethanol (150 mL) and water (150 mL). Magnesium sulfate (5.4 g) was then added, followed by potassium permanganate (7.2 g). The mixture was stirred at 25 °C for 1 hour, then extracted with ethyl acetate (600 mL). Organic layer was washed with brine (150 mL), dried over sodium sulfate, and solvent was removed under vacuum. The residue was purified by column chromatography (petroleum ether/ethyl acetate = 20/1 to 10/1). tert- Butyl 4-(2-ethoxy-2-oxo-ethyl)-4-fluoro-piperidine-l-carboxylate (0.6 g, 2.07 mmol, 11% yield) was obtained as a colorless oil.
References:
WO2021/11913,2021,A1 Location in patent:Paragraph 2284; 2285

79099-07-3
543 suppliers
$5.00/5g
![4-Piperidineacetic acid, 1-[(1,1-diMethylethoxy)carbonyl]-4-fluoro-, ethyl ester](/CAS/GIF/1235842-48-4.gif)
1235842-48-4
23 suppliers
inquiry