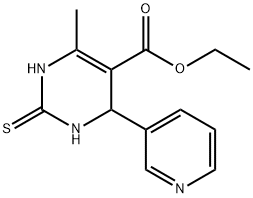
Pyrimidine-5-carboxylic acid, 1,2,3,4-tetrahydro-6-methyl-4-(3-pyridyl)-2-thioxo-, ethyl ester synthesis
- Product Name:Pyrimidine-5-carboxylic acid, 1,2,3,4-tetrahydro-6-methyl-4-(3-pyridyl)-2-thioxo-, ethyl ester
- CAS Number:123629-47-0
- Molecular formula:C13H15N3O2S
- Molecular Weight:277.34
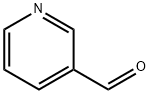
500-22-1
465 suppliers
$6.00/5g
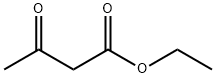
141-97-9
783 suppliers
$5.00/25g

17356-08-0
3 suppliers
inquiry

123629-47-0
11 suppliers
inquiry
Yield:123629-47-0 85%
Reaction Conditions:
with ammonium cerium (IV) nitrate in water at 70; for 0.5 h;Biginelli Pyrimidone Synthesis;
Steps:
General procedure for synthesis of pyrimidines 4a-g
General procedure: A mixture of aromatic aldehyde (pyridin-3-carboxaldehydeor thiophen-2-carboxaldehyde) (1mmol), active methylenecompounds (1mmol) namely ethyl acetoacetate, acetylacetoneand 1,3-diphenyl-1,3-propandione, urea dreivatives (urea, thiourea or guanidine hydrochloride) (1mmol), CAN(0.1mmol) in distilled water (10 mL) was heated and stirredunder reflux at 70 °C for 30min. The formed solid productwas filtered, washed with water, dried and recrystallized fromthe proper solvent to give pyrimidines 4a-g.
References:
Salem, Mounir A.;Behalo, Mohamed S.;Elrazaz, Eman [Medicinal Chemistry Research,2019,vol. 28,# 8,p. 1223 - 1234]