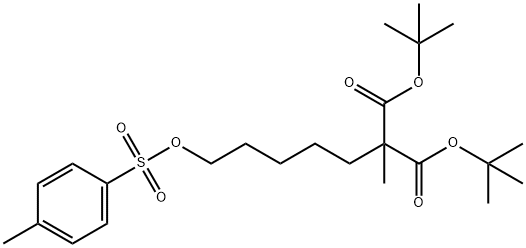
di-tert-butyl 2-Methyl-2-(5-(tosyloxy)pentyl)Malonate synthesis
- Product Name:di-tert-butyl 2-Methyl-2-(5-(tosyloxy)pentyl)Malonate
- CAS Number:1236354-13-4
- Molecular formula:C24H38O7S
- Molecular Weight:470.62
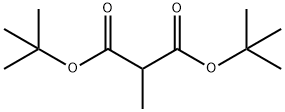
34812-95-8
13 suppliers
$45.00/10mg
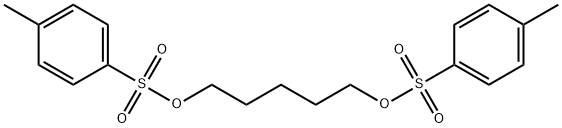
24293-28-5
10 suppliers
inquiry

1236354-13-4
11 suppliers
inquiry
Yield:1236354-13-4 57%
Reaction Conditions:
with sodium hydride in tetrahydrofuran at 50; for 16 h;Product distribution / selectivity;Inert atmosphere;
Steps:
2
Example 22-methyl di-tert butyl malonate (45 g, 0.2 mol) and 1,5 bis(p-toylsulfonato) pentane (322 g, 0.78 mol, 4 eq) were dissolved, under an argon atmosphere, in 1.6 L dry tetrahydrofuran (THF). Sodium hydride (NaH) (5.6 g, 1.2 eq) was added in one portion. The reaction mixture was heated up to 50° C. and stirring was maintained for 16 hrs at this temp. The mixture was filtered off, evaporated to dryness, diluted with tert-butyl methyl ether (TBME, 200 ml), filtered and evaporated again. The residue was taken up in TBME (100 ml) and was cooled in the refrigerator. The ditosylate compound crystallized out and was filtered off. The filtrate was evaporated to dryness affording (89 g, 97%) of the crude material as oil which solidified upon storage. Final purification was achieved by filtering the material through silica gel in a fritted funnel (Petroleum Ether/Ethyl Acetate 15:1/4:1) and further crystallization from ethanol to afford 50.5 g (57%) of a white pure solid having a melting point of 36.5-38.5° C.Mass spectrometry (Finnigan Surveyor MSQ Plus (APCl, neg.) results of the 2-(5 substituted-alkyl)-2-methyl malonic acid derivative showed m/z=469.3 [M-H]-.1H-NMR [Bruker Avance 400 (400 MHz, CDCl3, TMS as internal standard] of the 2-(5 substituted-alkyl)-2-methyl malonic acid derivative showed the following results: δ(ppm)=1.09-1.23 (m, 2H), 1.26 (s, 3H, Me), 1.27-1.36 (m, 2H), 1.43 (s, 18H, tBu), 1.57-1.74 (m, 4H), 2.45 (s, 3H, Me), 4.01 (t, 2H, j=6.5 Hz, CH2), 7.34 (d, 2H, J=8.1 Hz, Ph) 7.78 (d, 2H, J=8.3 Hz, Ph).13C-NMR [Bruker Avance 400 (100.6 MHz, CDCl3, TMS as internal standard] of the 2-(5 substituted-alkyl)-2-methyl malonic acid derivative showed the following results: δ(ppm)=19.7, 21.6, 23.6, 25.8, 27.9 (6C), 28.6, 36.1, 54.5, 70.4, 80.9 (2C), 127.9 (2C), 129.9 (2C), 133.3, 144.7, 171.7 (2C).IR (Bio-Rad FTS 3000MX (KBr)) results of the 2-(5 substituted-alkyl)-2-methyl malonic acid derivative showed: ν (cm-1)=3454 (w, br), 3005 (w), 2990 (w), 2973 (m), 2952 (m), 2935 (m), 2868 (w), 1748 (m), 1725 (s), 1599 (w), 1466 (m), 1394 (w), 1371 (m), 1358 (m), 1309 (m), 1292 (m), 1278 (m), 1255 (m), 1239 (m), 1221 (w), 1179 (vs), 1156 (m), 1123 (m), 1119 (m), 1098 (m), 1043 (w), 1019 (w), 970 (m), 946 (m), 920 (w), 904 (m), 867 (w), 851 (w), 829 (m), 811 (m), 767 (m), 725 (w), 706 (w), 666 (m), 579 (m), 555 (m), 506 (w), 486 (w), 469 (w).
References:
US2012/29223,2012,A1 Location in patent:Page/Page column 4