
(R)-3-(((benzyloxy)carbonyl)amino)-4-hydroxybutanoic acid synthesis
- Product Name:(R)-3-(((benzyloxy)carbonyl)amino)-4-hydroxybutanoic acid
- CAS Number:123673-31-4
- Molecular formula:C12H15NO5
- Molecular Weight:253.25
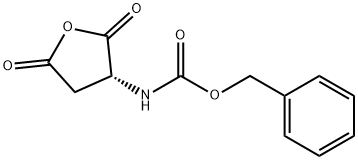
75443-62-8
35 suppliers
$112.00/100mg

123673-31-4
3 suppliers
inquiry
Yield:123673-31-4 85%
Reaction Conditions:
Stage #1: benzyl N-[(3R)-2,5-dioxotetrahydrofuran-3-yl]carbamatewith sodium tetrahydroborate in tetrahydrofuran at 0 - 25; for 6 h;
Stage #2: with hydrogenchloride;water in tetrahydrofuran at 0; pH=4;
Steps:
2; 4 (R)-3-(benzyloxycarbonylamino)-4-hydroxybutanoic acid
A solution of (R)-benzyl 2,5-dioxotetrahydrofuran-3-ylcarbamate (INTERMEDIATE 1, 53.28 g, 213.79 mmol) in THF (330 ml) was added over a 4 hour period using an additional funnel to a mixture of sodium borohydride (8.09 g, 213.79 mmol) in THF (330 ml) at 0° C. After the addition was complete, the mixture was warmed to room temperature and stirred for 2 hours. The solution was then concentrated under reduced pressure to ? of the original volume, cooled to 0° C., and carefully quenched by the slow addition of water (400 ml). The mixture was acidified to pH 4 with 1N aqueous hydrochloric acid and extracted with Et2O (5×300 ml). The combined organic layers were dried (Na2SO4) and concentrated in vacuo to provide the title product (45.9 g, yield: 85%).1H NMR (300 MHz, METHANOL-d4) δ ppm 2.41-2.54 (m, 1H) 2.56-2.70 (m, 1 H) 3.33 (dt, 2H) 3.42-3.65 (m, 2H) 3.95-4.12 (m, 1H) 5.01-5.22 (m, 2H) 7.14-7.46 (m, 5 H).LCMS: (ESI) m/z 254.1 [M+H]+.Optical Rotation:Concentration: 0.14 g/dLLamp: SodiumWavelength: 589 nmTemperature: 20° C.Path length: 10 cm Cell volume: 1 ml Solvent: CH2Cl2 [α]=+13
References:
US2012/35134,2012,A1 Location in patent:Page/Page column 9; 32
78663-07-7
226 suppliers
$5.00/1g

123673-31-4
3 suppliers
inquiry
87219-29-2
129 suppliers
$7.00/100mg

123673-31-4
3 suppliers
inquiry
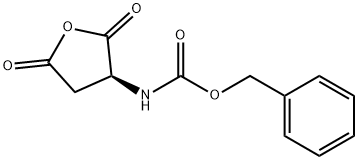
4515-23-5
65 suppliers
$15.00/1g

123673-31-4
3 suppliers
inquiry
![Carbamic acid, [1-[1-[[(1,1-dimethylethoxy)carbonyl]amino]-2-hydroxyethyl]-3-oxo-3-[(phenylmethyl)amino]propyl]-, phenylmethyl ester, [S-(R*,S*)]- (9CI)](/CAS/20210305/GIF/135517-09-8.gif)
135517-09-8
0 suppliers
inquiry

123673-31-4
3 suppliers
inquiry