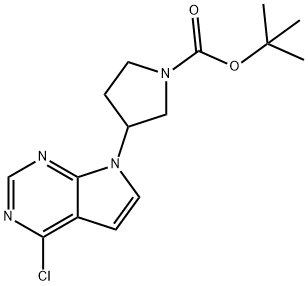
tert-Butyl 3-(4-chloro-7H-pyrrolo[2,3-d]pyrimidin-7-yl)pyrrolidine-1-carboxylate synthesis
- Product Name:tert-Butyl 3-(4-chloro-7H-pyrrolo[2,3-d]pyrimidin-7-yl)pyrrolidine-1-carboxylate
- CAS Number:1240301-66-9
- Molecular formula:C15H19ClN4O2
- Molecular Weight:322.79
![4-Chloro-7H-pyrrolo[2,3-d]pyrimidine](/CAS/GIF/3680-69-1.gif)
3680-69-1
802 suppliers
$6.00/1g

103057-45-0
42 suppliers
$105.00/50mg
![tert-Butyl 3-(4-chloro-7H-pyrrolo[2,3-d]pyrimidin-7-yl)pyrrolidine-1-carboxylate](/CAS/20200401/GIF/1240301-66-9.gif)
1240301-66-9
8 suppliers
inquiry
Yield: 45%
Reaction Conditions:
with potassium carbonate in dimethyl sulfoxide at 80;
Steps:
13
Reference Example 13 tert-butyl 3-(4-chloro-7H-pyrrolo[2,3-d]pyrimidin-7-yl)pyrrolidine-1-carboxylate; A mixture of 4-chloro-7H-pyrrolo[2,3-d]pyrimidine (1.01 g, 6.58 mmol), tert-butyl 3-[(4-methylphenyl)sulfonyl]oxy}pyrrolidine-1-carboxylate (2.70 g, 7.91 mmol) and potassium carbonate (3.64 g, 26.34 mmmol) in dimethyl sulfoxide (15 mL) was stirred at 80°C overnight. The reaction mixture was allowed to cool to room temperature, water was added thereto, and the resulting solid was removed by filtration. The filtrate was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate and filtered. The filtrate was concentrated. The obtained residue was subjected to silica gel column chromatography (hexane-ethyl acetate 95:5 - 60:40, v/v) and crystallized from hexane-ethyl acetate to give the title compound (953 mg, yield 45%) as colorless crystals. 1H-NMR (300 MHz, CDCl3)δ:1.49 (br s, 9 H), 2.08 - 2.38 (m, 1 H), 2.37 - 2.62 (m, 1 H), 3.15 - 3.87 (m, 3 H), 3.83 - 4.09 (m, 1 H), 5.25-5.69 (m, 1 H), 6.65 (d, J = 3.8 Hz, 1 H), 7.28 (br s, 1 H), 8.64 (s, 1 H).
References:
Takeda Pharmaceutical Company Limited EP2399914, 2011, A1 Location in patent:Page/Page column 93