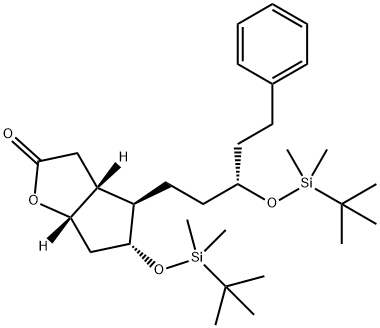
1240483-21-9 synthesis
- Product Name:1240483-21-9
- CAS Number:1240483-21-9
- Molecular formula:C30H52O4Si2
- Molecular Weight:532.9
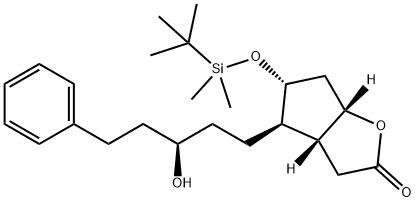
1240483-20-8
1 suppliers
inquiry

18162-48-6
664 suppliers
$9.00/5g

1240483-21-9
12 suppliers
inquiry
Yield:1240483-21-9 95%
Reaction Conditions:
with 1H-imidazole in dichloromethane at 20; for 18 h;Inert atmosphere;
Steps:
4
Preparation of the compound XIIImidazole (528 mg, 7.7 mmoles, 2.5 eq) and TBS-Cl (536 mg, 3.5 mmoles, 1.15 eq) are added, in the above order, at room temperature to a solution of the alcohol XI (1.3 g, 3.1 mmoles) in dicbloromethane (35 mL). The formation of a white precipitate can be immediately noted, the reaction is performed under stirring at room temperature and is complete after 18 hours. To quench the reaction, a saturated solution of sodium bicarbonate (25 mL) is added, it is diluted with dichloromethane (20 mL), the phases are separated, the aqueous phase is extracted with dichloromethane, the re-combined organic phases are dried on magnesium sulphate and filtered, and lastly the solvent is removed at reduced pressure. The product is purified by means of column chromatography (hexane- AcOEt 9:1 v/v) and the pure product is obtained as a white solid with a yield of 95%.
References:
WO2010/97672,2010,A1 Location in patent:Page/Page column 20-21

18162-48-6
664 suppliers
$9.00/5g
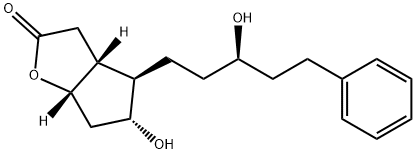
145667-75-0
93 suppliers
$100.00/10mg

1240483-21-9
12 suppliers
inquiry
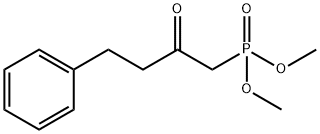
41162-19-0
175 suppliers
inquiry

1240483-21-9
12 suppliers
inquiry

64091-14-1
21 suppliers
$179.00/500mg

1240483-21-9
12 suppliers
inquiry
![(3aR,4R,5R,6aS)-5-((tert-butyldimethylsilyl)oxy)-4-((E)-3-hydroxy-5-phenylpent-1-en-1-yl)hexahydro-2H-cyclopenta[b]furan-2-one](/CAS/20200401/GIF/1240483-14-0.gif)
1240483-14-0
4 suppliers
inquiry

1240483-21-9
12 suppliers
inquiry