
ethyl 6-chloro-2-(pyridin-2-yl)pyrimidine-4-carboxylate synthesis
- Product Name:ethyl 6-chloro-2-(pyridin-2-yl)pyrimidine-4-carboxylate
- CAS Number:1240597-44-7
- Molecular formula:C12H10ClN3O2
- Molecular Weight:263.68

1240596-40-0
6 suppliers
inquiry

1240597-44-7
14 suppliers
inquiry
Yield:1240597-44-7 20%
Reaction Conditions:
Stage #1: ethyl 6-hydroxy-2-(2-pyridinyl)-4-pyrimidinecarboxylatewith trichlorophosphate at 90; for 2 h;
Stage #2: with sodium acetate in water at 20;
Steps:
Intermediate 109: ethyl 6-chloro-2-(2-pyridinyl)-4-pyrimidinecarboxylateA suspension of ethyl 6-hydroxy-2-(2-pyridinyl)-4-pyrimidinecarboxylate (2.91 g, 1 1 .87 mmol) in phosphorus oxychloride (6.08 mL, 65.3 mmol) was heated to 90 °C for 2 h. The reaction mixture was cooled to room temperature and then cautiously added to a stirring solution of sodium acetate (89 g, 653 mmol) in water (350 mL). The quenched reaction mixture was diluted with 10% MeOH/DCM (200mL) and the layers separated. The aqueous phase was further extracted with 10% MeOH/DCM (2 x 150mL) and the organic phase was dried (Na2S04), filtered and concentrated in vacuo to give a brown oil. The crude product was purified by column chromatography using a gradient of 0 to 75% EtOAc/cyclohexane to give the title compound as a beige solid (631 mg, 20 % yield)LCMS (Method A): Rt = 0.85 min, MH+ = 264.0
References:
WO2012/52390,2012,A1 Location in patent:Page/Page column 84-85
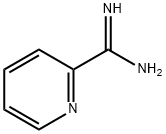
52313-50-5
38 suppliers
$121.00/1g

1240597-44-7
14 suppliers
inquiry
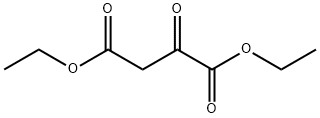
108-56-5
156 suppliers
$40.00/1g

1240597-44-7
14 suppliers
inquiry