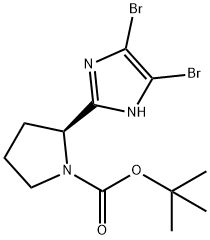
tert-butyl 2-(4,5-dibromo-1H-imidazol-2-yl)pyrrolidine-1-carboxylate synthesis
- Product Name:tert-butyl 2-(4,5-dibromo-1H-imidazol-2-yl)pyrrolidine-1-carboxylate
- CAS Number:1240893-76-8
- Molecular formula:C12H17Br2N3O2
- Molecular Weight:395.09
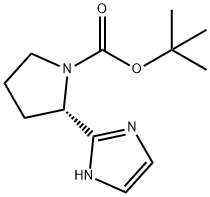
1007882-58-7
58 suppliers
$65.00/100mg

1240893-76-8
14 suppliers
inquiry
Yield:1240893-76-8 65%
Reaction Conditions:
with N-Bromosuccinimide in dichloromethane at 0; for 2 h;Product distribution / selectivity;
Steps:
Intermediate 1C; (S)-tert-buty 2-(4,5 -dibromo- l f-imidazol-2-yl)pyrrolidine- 1 -carboxylate; A -Bromosuccinimide (108 mmol) was added to a cold (0 °C) solution of the product from Intermediate IB (12.05 g, 50.8 mmol) in dichloromethane (200 mL). The mixture was stirred in ice bath for 2 hours and then concentrated, dissolved in ethyl acetate (250 mL), washed with water (3 x 150 mL) and brine (1 x 100 mL), dried (MgS04), and concentrated to very dark residue. The residue was mixed with and concentrated from dichloromethane/hexanes (1 : 1) to get brown solid (~19 g). The solid was triturated with ether (-100 mL) and filtered to isolate a tan solid (13.23 g, 65% yield). .H NMR (400 MHz, CDC13) δ ppm 1.49 (s, 9 H), 1.86 - 2.17 (m, 3 H), 2.80 - 2.95 (m, 1 H), 3.30 - 3.44 (m, 2 H), 4.85 (dd, J=7.54, 2.55 Hz, 1 H), 10.82 (s, 1 H); MS (DCI+) m/z 394/396/398 (M+H)+.
References:
WO2012/83170,2012,A1 Location in patent:Page/Page column 151
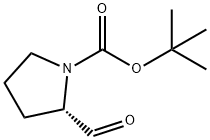
69610-41-9
250 suppliers
$9.00/1g

1240893-76-8
14 suppliers
inquiry
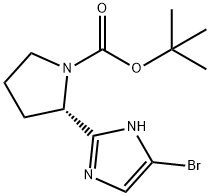
1007882-59-8
96 suppliers
$24.00/250mg

1240893-76-8
14 suppliers
inquiry
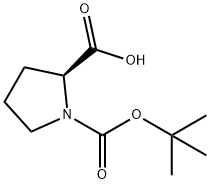
15761-39-4
602 suppliers
$5.00/10g

1240893-76-8
14 suppliers
inquiry
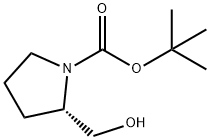
69610-40-8
382 suppliers
$6.00/5g

1240893-76-8
14 suppliers
inquiry