TERT-BUTYL 2-(3-OXOCYCLOHEXYL)-4-PHENYLPIPERIDINE-1-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 2-(3-OXOCYCLOHEXYL)-4-PHENYLPIPERIDINE-1-CARBOXYLATE
- CAS Number:1241505-11-2
- Molecular formula:C22H31NO3
- Molecular Weight:357.49
Yield:1241505-11-2 46%
Reaction Conditions:
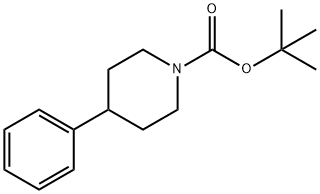
Stage #1: tert-butyl 4-phenylpiperidine-1-carboxylatewith N,N,N,N,-tetramethylethylenediamine;sec.-butyllithium in diethyl ether at -78; for 2 h;
Stage #2: with copper(I) cyanide di(lithium chloride) in tetrahydrofuran at -50; for 1 h;
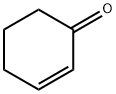
Stage #3: cyclohexenonewith chloro-trimethyl-silane;water in diethyl ether at -50 - 20;
Steps:
II.1
To a solution of compound 1 (10.8g, 41.2 mmol) in Et2O (150 mL) at -78 0C was added TMEDA (6.2mL, 41.2 mmol), followed by sec-BuLi (41.4mL, 1.3eq, 53.8 mmol). The mixture was stirred at -78 0C for 2 h before a solution of CuCN-2LiCl (3.6 g, 20.6 mmol) in THF (41.2 mL) was added. The reaction mixture was allowed to stir at -500C for 1 h before a solution of compound 2 (4g, 41.2 mmol) in TMSCl (26mL, 206 mmol) was added. The reaction mixture was stirred at - 500C for 30 min and then was allowed to stir at room temperature overnight. The reaction mixture was quenched with H2O, stirred at room temperature briefly, diluted with EA, and filtered through Celite. The organic phase was separated, and the aqueous phase was extracted with EA (40 mLχ4). The combined organic phases were washed with saturated NH4C1 (aq.), 5% NaHCO3 (aq.), brine, and dried over a mixture of anhydrous Na2SO4 and K2CO3. Evaporation of the solvent in vacuo afforded the crude product which was purified by column chromatography to give 6.8 g of compound 3 in 46 % yields.
References:
WO2010/94242,2010,A1 Location in patent:Preparation II.1