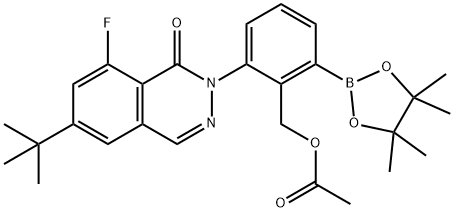
2-(6-(tert-butyl)-8-fluoro-1-oxophthalazin-2(1H)-yl)-6-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)benzyl acetate synthesis
- Product Name:2-(6-(tert-butyl)-8-fluoro-1-oxophthalazin-2(1H)-yl)-6-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)benzyl acetate
- CAS Number:1242156-76-8
- Molecular formula:C27H32BFN2O5
- Molecular Weight:494.36
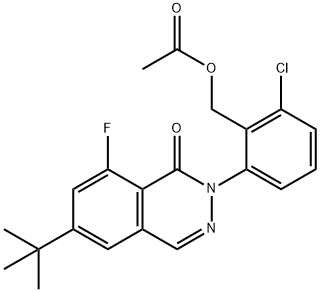
1242157-24-9
3 suppliers
inquiry

73183-34-3
584 suppliers
$6.00/5g

1242156-76-8
19 suppliers
$713.00/100mg
Yield:1242156-76-8 81%
Reaction Conditions:
Stage #1: 2-(6-tert-butyl-8-fluoro-1-oxophthalazin-2(1H)-yl)-6-chlorobenzyl acetate;bis(pinacol)diboranewith potassium acetate;XPhos in 1,4-dioxane; for 0.25 h;Inert atmosphere of argon;
Stage #2: bis(dibenzylideneacetone)-palladium(0) in 1,4-dioxane at 60; for 18 h;
Steps:
26
In a microwave reaction vial, added ccetic acid 2-(6-tert-butyl-8-fluoro-1-oxo-1H-phthalazin-2-yl)-6-chloro-benzyl ester (329 mg, 0.818 mmol), bis-pinaco-diboron (416 mg, 1.637 mmol), KOAc (241 mg, 2.454 mmol) and Xphos (39 mg, 0.0818 mmol) and dioxane (4 mL). Bubble argon through for 15 min and then add Pd(dba)2 (24 mg, 0.0409 mmol). Seal the tube and heat it to 60° C. for 18 hrs. The reaction mixture was then diluted with EtOAc (5 mL) and washed with NaHCO3 (concentrated) (1*10 mL) and water (10 mL). The organic phase was then concentrated and purified on silica gel column with 25% EtOAc in Hex to give acetic acid 2-(6-tert-butyl-8-fluoro-1-oxo-1H-phthalazin-2-yl)-6-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan -2-yl)-benzyl ester as an yellow oil (330 mg, 81%).
References:
US2010/222325,2010,A1 Location in patent:Page/Page column 69
![2-Bromo-6-(6-tert-butyl-8-fluoro-1-oxo-1,2-dihydrophthalazin-2-yl)phenyl]methyl acetate](/CAS/20210111/GIF/1413063-73-6.gif)
1413063-73-6
3 suppliers
inquiry

73183-34-3
584 suppliers
$6.00/5g

1242156-76-8
19 suppliers
$713.00/100mg
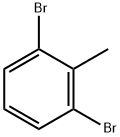
69321-60-4
199 suppliers
$11.00/1g

1242156-76-8
19 suppliers
$713.00/100mg
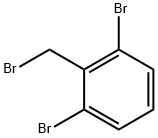
93701-32-7
54 suppliers
$501.91/5MG

1242156-76-8
19 suppliers
$713.00/100mg
1147858-83-0
33 suppliers
$228.00/250mg

1242156-76-8
19 suppliers
$713.00/100mg