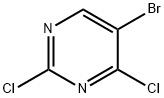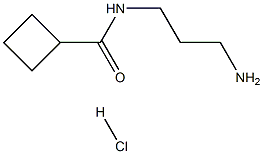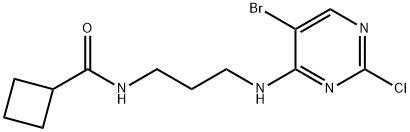
cyclobutanecarboxylic acid [3-(5-bromo-2-chloro-pyrimidin-4-ylamino)-propyl]-amide synthesis
- Product Name:cyclobutanecarboxylic acid [3-(5-bromo-2-chloro-pyrimidin-4-ylamino)-propyl]-amide
- CAS Number:1243268-69-0
- Molecular formula:C12H16BrClN4O
- Molecular Weight:347.64
Yield:1243268-69-0 71%
Reaction Conditions:
with triethylamine in 1,4-dioxane at 20; for 6 h;
Steps:
To a suspension of cyclobutanecarboxylic acid (3-amino-propyl)-amide hydrochloride (2.55 g, 13.23 mmol) in dioxane (50 mL) was added triethylamine (9.22 ml_, 66.2 mmol) followed by 2,4-dichloro-5-bromopyrimidine (2.51 g, 11.03 mmol), and the reaction was stirred at room temperature for 6 h. The dioxane was removed in vacuo and the residue partitioned between water (40 mL) and EtOAc (40 mL). The aqueous layer was re- extracted with EtOAc (2 x 30 mL) and the combined organic extracts washed with water (70 mL) and brine (70 mL), dried (MgSO4) and concentrated in vacuo. Purification by flash chromatography using a Biotage SP4 (40-60% petroleum ether-EtOAc gradient) gave the product as a white solid (2.71 g, 71%). δH (400 MHz, d6-DMSO) 8.23 (s, 1H), 7.75 (t, J = 5.5 Hz, 1H), 7.69 (t, J = 5.5 Hz, 1H)1 3.33 (q, J = 6.4 Hz, 2H), 3.05 (q, J = 6.4 Hz, 2H)1 2.96 (quintet, J = 8.2 Hz1 1H)1 2.16-1.59 (m, 8H); m/z (ES+APCI)+: 347 / 349 / 351 [M+H]+.
References:
WO2010/100431,2010,A1 Location in patent:Page/Page column 45