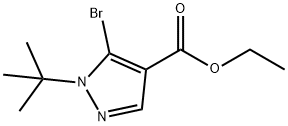
ethyl 5-bromo-1-tert-butyl-1H-pyrazole-4-carboxylate synthesis
- Product Name:ethyl 5-bromo-1-tert-butyl-1H-pyrazole-4-carboxylate
- CAS Number:1245272-40-5
- Molecular formula:C10H15BrN2O2
- Molecular Weight:275.14
112779-14-3
56 suppliers
$9.00/100mg

1245272-40-5
12 suppliers
inquiry
Yield:1245272-40-5 90%
Reaction Conditions:
with n-Amyl nitrite;copper(ll) bromide in acetonitrile at 20; for 4 h;
Steps:
39.2
(step 2)
To a solution of pentyl nitrite (16.64 g, 142.00 mmol) and copper(II) bromide (5.32 mL, 113.60 mmol) in acetonitrile (100 mL) was added dropwise a solution of the compound (20 g, 94.67 mmol) obtained in step 1 in acetonitrile (50 mL), and the mixture was stirred at room temperature for 4 hr.
Then, 6N hydrochloric acid was added thereto, and the mixture was extracted with ethyl acetate.
The organic layer was washed with water, aqueous sodium hydrogen carbonate solution and saturated brine and dried, and the solvent was evaporated under reduced pressure.
The residue was purified by silica gel column chromatography (solvent gradient; 2→30% ethyl acetate/hexane) to give ethyl 5-bromo-1-tert-butyl-1H-pyrazole-4-carboxylate (23.5 g, 85 mmol, 90%) as a brown oil. 1H-NMR(300MHz,CDCl3):δ1.35(3H, t, J=7.19Hz), 1.77(9H, s), 4.31(2H, q, J=7.19Hz), 7.87(1H, s)
References:
EP2738170,2014,A1 Location in patent:Paragraph 0181; 0348
94-05-3
268 suppliers
$5.00/5g

1245272-40-5
12 suppliers
inquiry