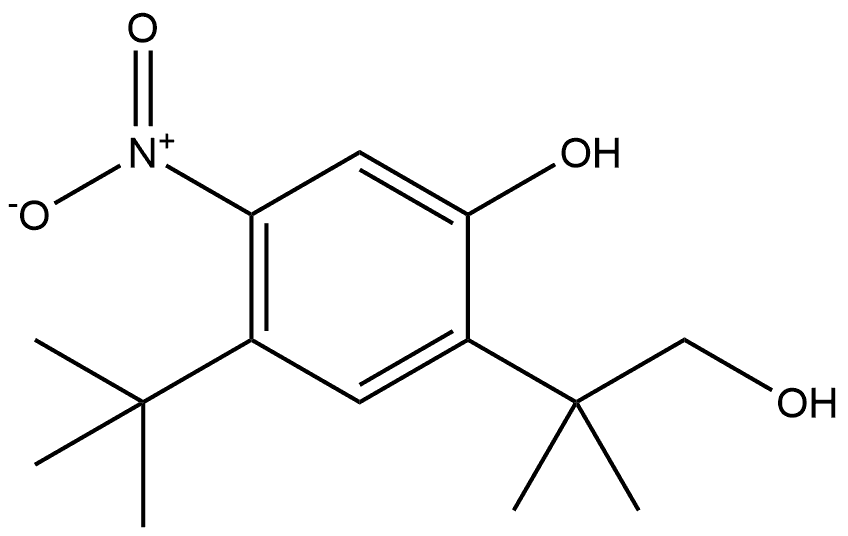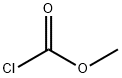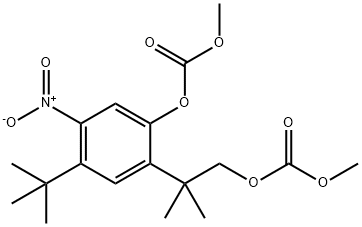
Carbonic acid, 4-(1,1-diMethylethyl)-2-[2-[(Methoxycarbonyl)oxy]-1,1-diMethylethyl]-5-nitrophenyl Methyl ester synthesis
- Product Name:Carbonic acid, 4-(1,1-diMethylethyl)-2-[2-[(Methoxycarbonyl)oxy]-1,1-diMethylethyl]-5-nitrophenyl Methyl ester
- CAS Number:1246213-36-4
- Molecular formula:C18H25NO8
- Molecular Weight:383.39
Yield:-
Reaction Conditions:
with triethylamine;dmap at 0 - 20;
Steps:
2
To a solution of 4-tert-butyl-2-( 1 -hydroxy-2-methylpropan-2-yl)-5-nitrophenol (1.92 g, 7.18 mmol), triethylamine (1.745 g, 17.24 mmol), and dimethylaminopyridine (87.74 mg, 0.718 mmol) in dichloromethane (30 mL) at 0 0C was slowly charged methylchloroformate (2.376 g, 25.14 mmol), keeping the temperature below 5 0C. After the addition, the mixture was allowed to warm to ambient temperature and was stirred until HPLC showed complete conversion of the starting material (2-8 h). The reaction mixture was diluted with water and acidified with IN HCl (pH 1-2). The aqueous phase was extracted with DCM and the combined organics dried in vacuo. The crude amber semi-solid was re- crystallized from methanol and dichloromethane to give the title compound as a yellow crystalline solid. 1H-NMR (400 MHZ, DMSO-^6) δ 7.67 (s, IH); 7.52 (s, IH); 4.30 (s, 2H); 3.86 (s, 3H); 3.64 (s, 3H); 1.35 (s, 9H); 1.35 (s, 6H)
References:
WO2010/108155,2010,A1 Location in patent:Page/Page column 42-44
![Carbonic acid, 4-(1,1-diMethylethyl)-2-[2-[(Methoxycarbonyl)oxy]-1,1-diMethylethyl]phenyl Methyl ester](/CAS/GIF/1246213-31-9.gif)
1246213-31-9
0 suppliers
inquiry
![Carbonic acid, 4-(1,1-diMethylethyl)-2-[2-[(Methoxycarbonyl)oxy]-1,1-diMethylethyl]-5-nitrophenyl Methyl ester](/CAS/GIF/1246213-36-4.gif)
1246213-36-4
0 suppliers
inquiry