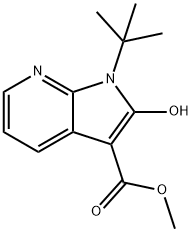
methyl 1-tert-butyl-2-hydroxy-1H-pyrrolo[2,3-b]pyridine-3-carboxylate synthesis
- Product Name:methyl 1-tert-butyl-2-hydroxy-1H-pyrrolo[2,3-b]pyridine-3-carboxylate
- CAS Number:1246733-24-3
- Molecular formula:C13H16N2O3
- Molecular Weight:248.28
![potassiuM 1-tert-butyl-3-(Methoxycarbonyl)-1H-pyrrolo[2,3-b]pyridin-2-olate](/CAS/GIF/1246647-79-9.gif)
1246647-79-9
2 suppliers
inquiry
![methyl 1-tert-butyl-2-hydroxy-1H-pyrrolo[2,3-b]pyridine-3-carboxylate](/CAS/20180703/GIF/1246733-24-3.gif)
1246733-24-3
4 suppliers
inquiry
Yield:1246733-24-3 100%
Reaction Conditions:
with acetic acid in methanol at 6; for 0.0833333 h;Industry scale;
Steps:
1.A2.3
Step A2 -One Pot Preparation of Ox-indole Methyl EsterMaterial FW mol AmountStep (1):2-tButylamino3 -methyl pyridine 7 164.25 1 26.36 4.33 kgn-Hexyl Lithium (at) 2.09 M/hexane 1.0 26.36 12.60 LMethyl chloroformate, 98% 94.50/dl.228 1.04 26.36 2.13 LTHF 28+6 LStep (2):Hexyllithium (at) 2.09 M 0.7 18.45 9.10 LDiisopropylamine, 99% 101.2Λ10.716 1.4 36.90 5.27 LHexyllithium (at) 2.09 M 1.6 39.54 20.9 L Step (3):Methyl Chloroformate, 98% 94.5/dl.228 1.3 34.27 2.66 LWork-up:HCl, 5.0N 14.5 LKOH, 4.0 N 4 LK2CO3, 22% (made from K2CO3 99%) 12.6LStep (1):To a 4-necked RB flask (100L) was charged a soln of 7 (~28L, 17.5%wt, 24.7Kg, 23.36 mol) under N2 and extra THF (6L) was added. This solution was cooled to <-10 0C with dry-ice acetone bath (external temp (at)-35 0C). To this solution was added n-hexyllithium (12.6 L, 2.09 M in hexane, 1.0 eq.) via addition funnel while controlling temp<-5 0C over 45 min(exothermic) .After addition, reaction was aged for 20min at the temp of -6 to -8°C. Methyl Chloroformate (2.05L, 1.00 equiv.) was slowly added via addition funnel over 43 min (exothermic, internal temp was controlled <14.2 0C); 10 min after addition (when temp became steady), the reaction was aged for about 1.5 h at 15 to 20 0C ((at)92%conversion based on the ratio of LCAP). Another 0.04 eq. (82 ml) of Methyl chloroformate was added to get about 94% conversion (before addition, temp was about 15 0C).Step (2):The above reaction was cooled to below -18 to -25 0C; 0.7 eq of hexyllithium(9.10 L, 2.09 N) was added (very exothermic - controlled internal temp at -15 0C to -20 OC) and the reaction was aged at -15 0C to -20 oC for 30 min. DIPA (5.27L, 1.4 eq) followed by hexyllithium (20.9 L, 2.09 N, 1.6 equiv.) was added via an additional funnel (controlling temp -15 0C to -20 0C during addition). The reaction was then aged for 20 min at <-15 0C (temp became steady), then changed to ice-water bath and temp was gradually warmed to 15 0C during overnight aging.Step (3): After the above reaction completed, it was cooled to 0 0C with dry ice-acetone bath and methyl chloro formate (2.66 L, 1.3 equiv.) was added via an addition funnel over 25 min (controlling temp below 13 0C during addition. Reaction went from a dark solution to an orange slurry).Reaction was aged for 40 min around 8 to 10 0C and LC showed complete conversion.Work-upTHF (6.4 L) was charged. The reaction was quenched with 5N HCl (14.5 L) at <20 0C over 2h to pH 4.28 (mixed soln), pH 3.88 (aq layer). Some precipitate was formed. 5L of water was added and the precipitate was dissolved. This mixture was transferred into an extractor. The aqueous layer (30 L) was cut; the organic was washed with water (8L). (The organic soln could be stored at RT under N2 for 2 days, but extended storage could result in decarboxylation.)The organic layer was then transferred to a 100 L RB flask and concentrated to give 2OL of a slurry.To the concentrated batch was charged THF (30L) and heptane (6L), and this soln. was transferred to a 100 L extractor containing 12.6L 22wt/wt% aq K2CO3 soln, cooled to10 0C, The pH was adjusted to 13.1 (suggest PH range: 12.5 to 13.5) with KOH (4 L, 4.0 N). After warming up to 16 to 20 0C, the aq layer was cut (no loss); the organic layer was washed with 15% K2CO3 (9L) (15% K2CO3 was used to wash out residual KOH which could decompose the K-salt) and transferred to 100 RB flask (this soln was stable at rt/N2 for overnight). The batch was concentrated and flushed with 80 L THF to remove water.To the concentrated batch (~ total 10 L with 2.5 L THF content) was charged 2L more THF.The product had begun to crystallize out and 60 L of n-heptane (6.7% loss in supernatant with only 30 L heptane) was charged via additional funnel in about 60 min. The resulting pale yellow slurry was aged for ca. 2 h (overnight aging is fine too). The product was collected by filtration, rinsed with 13 L of a solution of THF/heptane (1 :13) and then 10 L heptane. The pale yellow product was dried under vacuum/N2. to give total 5.0 kg of the K-salt was obtained (>99% LCAP, 94%wt, 63%yield) and with 5.6% loss in the mother liquid.Salt break and neutral form isolationIn a 100 L R.B. flask, charge K-salt (4.565 kg, 15.94 mol), to 16 L methanol, chill in ice bath to 60C. Add acetic acid (1.369 L, 23.91 mol) over 5 min. Near the end of addition, the mixture became very thick. Add 48 L water, age 1.5 hours at 15-2O0C (pH reading 6.1). Filter off solid, wash cake with 15 L water. Sucked dry on filter pot with N2 stream. Obtained 4.16 kg (105% uncorrected for residual water) as a free-flowing off- white powder.
References:
WO2011/5731,2011,A2 Location in patent:Page/Page column 55-58
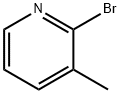
3430-17-9
448 suppliers
$4.00/5g
![methyl 1-tert-butyl-2-hydroxy-1H-pyrrolo[2,3-b]pyridine-3-carboxylate](/CAS/20180703/GIF/1246733-24-3.gif)
1246733-24-3
4 suppliers
inquiry

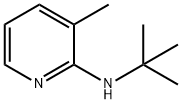
![lithium 1-(tert-butyl)-1H-pyrrolo[2,3-b]pyridin-2-olate](/CAS/20180601/GIF/1321609-73-7.gif)