
(3aR,4S,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-4-[[[(1,1-dimethylethyl)diphenylsilyl]oxy]methyl]tetrahydro-3-(phenylmethyl)furo[3,4-d]oxazol-2(3H)-one synthesis
- Product Name:(3aR,4S,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-4-[[[(1,1-dimethylethyl)diphenylsilyl]oxy]methyl]tetrahydro-3-(phenylmethyl)furo[3,4-d]oxazol-2(3H)-one
- CAS Number:125127-12-0
- Molecular formula:C34H36N6O4Si
- Molecular Weight:620.77
![9H-Purin-6-amine, 9-[3-bromo-3-deoxy-5-O-[(1,1-dimethylethyl)diphenylsilyl]-2-O-[[(phenylmethyl)amino]carbonyl]-β-D-xylofuranosyl]-](/CAS/20210305/GIF/125084-69-7.gif)
125084-69-7
1 suppliers
inquiry
![(3aR,4S,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-4-[[[(1,1-dimethylethyl)diphenylsilyl]oxy]methyl]tetrahydro-3-(phenylmethyl)furo[3,4-d]oxazol-2(3H)-one](/CAS/20210305/GIF/125127-12-0.gif)
125127-12-0
2 suppliers
inquiry
Yield:125127-12-0 315 g
Reaction Conditions:
with sodium t-butanolate in tetrahydrofuran at -20; for 0.5 h;
Steps:
(3aR,4S,6R,6aR)-6-(6-amino-9H-purin-9-yl)-3-benzyl-4-((tert- butyldiphenylsilyloxy)methyl)-tetrahydrofuro[3,4-d]oxazol-2(3H)-one (105)
: A solution of (2R,3S,4S,5R)-2-(6-amino-9H-purin-9-yl)-4-bromo-5-((tert- butyldiphenylsilyloxy)methyl)-tetrahydrofuran-3-yl benzylcarbamate (12, 348 g, 0.50 mol) in tetrahydrofuran (10.5 L) was treated with sodium tert-butoxide (57.2 g, 0.60 mol) for 0.5 h at -20 oC. The reaction was then quenched by the addition of saturated aqueous ammonium chloride (4 L). The organic phase was separated and the aqueous phase was extracted with ethyl acetate (2 L). The combined organic layers were dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure to afford the title compound 13 which was used in the next step without further purification (315 g, white foam): LC/MS: [(M + 1)]+ = 621.2.
References:
WO2018/45204,2018,A1 Location in patent:Page/Page column 84; 87
![9H-Purin-6-amine, 9-[3-bromo-3-deoxy-5-O-[(1,1-dimethylethyl)diphenylsilyl]-β-D-xylofuranosyl]-](/CAS/20210305/GIF/125084-68-6.gif)
125084-68-6
1 suppliers
inquiry
![(3aR,4S,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-4-[[[(1,1-dimethylethyl)diphenylsilyl]oxy]methyl]tetrahydro-3-(phenylmethyl)furo[3,4-d]oxazol-2(3H)-one](/CAS/20210305/GIF/125127-12-0.gif)
125127-12-0
2 suppliers
inquiry

58479-61-1
405 suppliers
$10.00/25g
![(3aR,4S,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-4-[[[(1,1-dimethylethyl)diphenylsilyl]oxy]methyl]tetrahydro-3-(phenylmethyl)furo[3,4-d]oxazol-2(3H)-one](/CAS/20210305/GIF/125127-12-0.gif)
125127-12-0
2 suppliers
inquiry
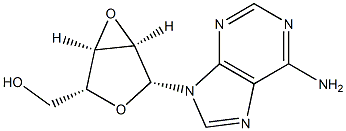
2627-64-7
11 suppliers
inquiry
![(3aR,4S,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-4-[[[(1,1-dimethylethyl)diphenylsilyl]oxy]methyl]tetrahydro-3-(phenylmethyl)furo[3,4-d]oxazol-2(3H)-one](/CAS/20210305/GIF/125127-12-0.gif)
125127-12-0
2 suppliers
inquiry
125084-70-0
0 suppliers
inquiry
![(3aR,4S,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-4-[[[(1,1-dimethylethyl)diphenylsilyl]oxy]methyl]tetrahydro-3-(phenylmethyl)furo[3,4-d]oxazol-2(3H)-one](/CAS/20210305/GIF/125127-12-0.gif)
125127-12-0
2 suppliers
inquiry