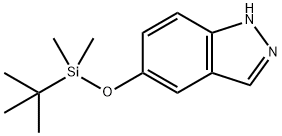
5-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-1H-Indazole synthesis
- Product Name:5-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-1H-Indazole
- CAS Number:1254473-74-9
- Molecular formula:C13H20N2OSi
- Molecular Weight:248.4
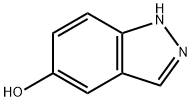
15579-15-4
208 suppliers
$5.00/250mg

18162-48-6
664 suppliers
$9.00/5g
![5-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-1H-Indazole](/CAS/20180703/GIF/1254473-74-9.gif)
1254473-74-9
11 suppliers
inquiry
Yield:1254473-74-9 100%
Reaction Conditions:
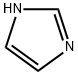
with 1H-imidazole in N,N-dimethyl-formamide at 0 - 18; for 3.5 h;
Steps:
5
Charge a 10 L reaction vessel with N,N-dimethylformamide (DMF, 2.50 L), 5 -hydroxy indazole (150.20 g, 1.12 mol) and lH-imidazole (114.35 g, 1.68 mol). Cool the mixture to 0 0C and add tert-butyldimethylchlorosilane (253.16 g, 1.68 mol) over 0.5 hours. Stir the mixture at 18 0C for 3 hours. Add water (2.5 L) to the reaction slowly with an ice bath at 5 0C to maintain an internal temperature at around 20 0C. Transfer the mixture to a separating funnel and extract with EA (2 x 2.5 L). Combine the extracts and wash with water (3 x 2.5 L) and brine. Dry the organic solutions over anhydrous sodium sulfate, filter, and evaporate to a red oil. Pass the oil through a silica gel pad and elute with eluent (0% to 30% EA in hexane) to afford the title compound as an orange oil which crystallizes. Yield: 300 g (100%). MS (ES) m/z 249 [M+l]+.
References:
WO2010/129509,2010,A1 Location in patent:Page/Page column 5
288-32-4
1005 suppliers
$5.00/25g

15579-15-4
208 suppliers
$5.00/250mg

18162-48-6
664 suppliers
$9.00/5g
![5-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-1H-Indazole](/CAS/20180703/GIF/1254473-74-9.gif)
1254473-74-9
11 suppliers
inquiry