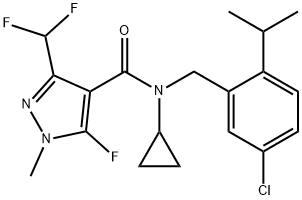
Isoflucypram synthesis
- Product Name: Isoflucypram
- CAS Number:1255734-28-1
- Molecular formula:C19H21ClF3N3O
- Molecular Weight:399.84
Yield:1255734-28-1 93%
Reaction Conditions:
in chlorobenzene at 100; for 8 h;Inert atmosphere;
Steps:
Preparation example: N-(2-isopropyl-5-chlorol-benzyl)-N-cyclopropyl-5-fluoro-l-methyl-3- difluoromethyl- 1 H-pyrazole-4-carboxamide
Under protective gas (argon), a solution of N-(2-isopropyl-5-chloro-benzyl) cyclopropylamine 22,4 g (lOO mmol) in 100 ml of chlorobenzene is initially charged. 21,2 g (lOO mmol) of l-methyl-3- difluoromethyl-5-fluoro-lH-pyrazole-4-carbonyl chloride are added and the mixture is stirred at 100°C for 8 h. For workup, the solvent was removed in vacuum and the residue was washed with 50 ml of cold isopropanol to give 37 g (93% of theory) carboxamide in the form of white crystals with melting point of 108-110°C.
References:
WO2014/76007,2014,A1 Location in patent:Page/Page column 6
![N-{[5-chloro-2-(propan-2-yl)phenyl]methyl}cyclopropanamine](/CAS/20200331/GIF/1476113-93-5.gif)