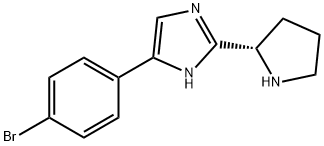
S)-2-((Methoxycarbonyl)aMino)-3-Methylbutanoic acid synthesis
- Product Name:S)-2-((Methoxycarbonyl)aMino)-3-Methylbutanoic acid
- CAS Number:1255936-24-3
- Molecular formula:C13H14BrN3
- Molecular Weight:292.17
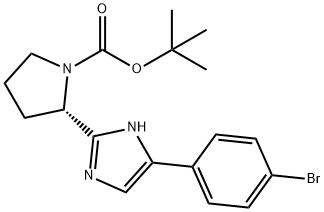
1007882-04-3
60 suppliers
inquiry

1255936-24-3
8 suppliers
inquiry
Yield:1255936-24-3 99%
Reaction Conditions:
with trifluoroacetic acid in dichloromethane at 0 - 20; for 5 h;
Steps:
3 Step 3: Synthesis of Compound BB-8
Compound BB-8-3 (3.0 g, 7.65 mmol) was dissolved in dichloromethane (60 mL) and cooled to 0° C., trifluoroacetic acid (20 mL) was dripped gradually, and the reaction was stirred at room temperature for 5 h. The reaction solution was concentrated under reduced pressure to remove the solvent by a rotary evaporator, the resulting oil was neutralized with saturated sodium bicarbonate solution (pH=8) and extracted with ethyl acetate (40 mL×2), the organic phases obtained twice were combined and dried over anhydrous sodium sulfate, the filtrate obtained after filtration was concentrated under reduced pressure to remove the solvent thereby delivering the title compound BB-8 (2.2 g, 99%). LCMS m/z: 292.0 [M+1]+
References:
US2017/253614,2017,A1 Location in patent:Paragraph 0188; 0191
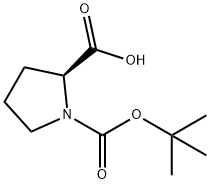
15761-39-4
611 suppliers
$5.00/10g

1255936-24-3
8 suppliers
inquiry
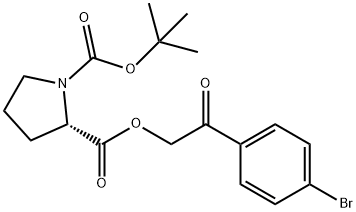
128120-93-4
1 suppliers
inquiry

1255936-24-3
8 suppliers
inquiry
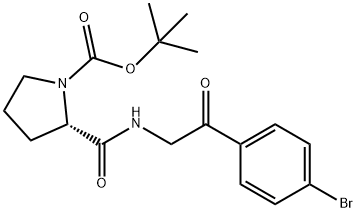
1007881-98-2
37 suppliers
inquiry

1255936-24-3
8 suppliers
inquiry
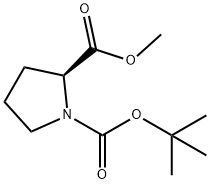
59936-29-7
226 suppliers
$6.00/5g

1255936-24-3
8 suppliers
inquiry