
4-(1-((tert-Butoxycarbonyl)amino)cyclopropyl)benzoic acid synthesis
- Product Name:4-(1-((tert-Butoxycarbonyl)amino)cyclopropyl)benzoic acid
- CAS Number:1256336-73-8
- Molecular formula:C15H19NO4
- Molecular Weight:277.32
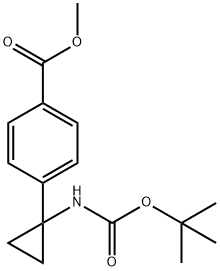
1338243-88-1
13 suppliers
inquiry

1256336-73-8
9 suppliers
inquiry
Yield:1256336-73-8 69%
Reaction Conditions:
with lithium hydroxide monohydrate;lithium hydroxide monohydrate in tetrahydrofuran;methanol at 20; for 16 h;Inert atmosphere;
Steps:
2 4-(7-bromobenzo[ d ]imidazo[ 2, 1 -b ]thiazol-2-yl)-3-( trifluoromethyl )benzoic acid
General procedure: To a stirred solution of methyl 4-(7-bromobenzo[d ]imidazo[2, 1 -b ]thiazol-2-yl)-3- (trifhioromethyl)benzoate (850 mg, 1.867 mmol) in methanol (10 mL) and water (3 mL) was added lithium hydroxide (224 mg, 9.353 mmol) at room temperature under a nitrogen atmosphere. The reaction solution was stirred for 16 h at room temperature. The resulting mixture was acidified to pH 6 with aqueous 2 M HC1 . The precipitated solids were collected by filtration, washed with dichloromethane (3 x 10 mL) and dried, to afford 4-(7-bromobenzo[d ]imidazo[2, 1 -ri]thiazol-2-yl )-3-(trifluoromethyl)benzoic acid as an off- white solid. Yield 430 mg (52%). 'H NMR (400 MHz, DMSO) S 13.50 (br s, 1H), 8.64 (s, 1H), 8.38 (d, J = 2.0 Hz, 1H), 8.31 (d, J = 1.6 Hz, 1H), 8.27 (dd, J = 2.0, 8.0 Hz, 1H), 8.18 (d, J = 8.4 Hz, 1H), 8.08 (d, J = 8.0 Hz, 1H), 7.77 (dd, J = 1.6, 8.4 Hz, 1H). m/z: [ESL] 441, 443 (M+H)+.
References:
WO2022/150316,2022,A1 Location in patent:Paragraph 00236; 00468-00469; 00594-00595
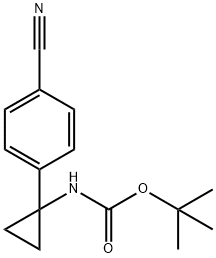
1338243-87-0
4 suppliers
inquiry

1256336-73-8
9 suppliers
inquiry
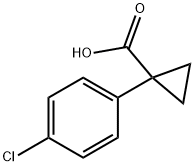
72934-37-3
104 suppliers
$11.00/1g

1256336-73-8
9 suppliers
inquiry
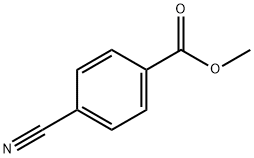
1129-35-7
306 suppliers
$8.00/5g

1256336-73-8
9 suppliers
inquiry
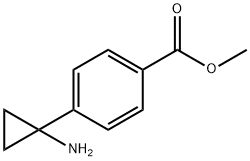
1006037-03-1
76 suppliers
$65.00/50mg

1256336-73-8
9 suppliers
inquiry