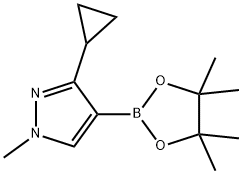
3-cyclopropyl-1-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrazole synthesis
- Product Name:3-cyclopropyl-1-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrazole
- CAS Number:1257637-82-3
- Molecular formula:C13H21BN2O2
- Molecular Weight:248.13
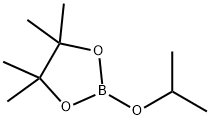
61676-62-8
313 suppliers
$11.19/5G
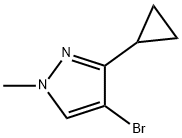
1257637-80-1
9 suppliers
$240.00/200mg

1257637-82-3
12 suppliers
inquiry
Yield:1257637-82-3 48%
Reaction Conditions:
Stage #1: 4-bromo-3-cyclopropyl-1-methyl-1H-pyrazolewith n-butyllithium in tetrahydrofuran at -78; for 0.5 h;
Stage #2: 2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane in tetrahydrofuran at 20; for 0.5 h;
Steps:
To a stirred solution of 4-bromo-3-cyclopropyl-l-methyl-lH-pyrazole (500 mg, 2.5 mmol) in TΗF (10 niL) was added dropwise a 2.5 M solution of n-BuLi (1.4 niL, 3.5 mmol) at -78 0C, and the resulting light yellow solution was stirred for 30 min. To the mixture was added a solution of 2-isopropoxy-4,4,5,5-tetramethyl-l,3,2-dioxaborolane (1.1 niL, 5.5 mmol) in TΗF (1 mL), and the mixture was warmed to room temperature over 30 min. The reaction was quenched with saturated aqueous NΗ4CI solution. The mixture was extracted with EtOAc (50 mL), washed with brine, dried over MgSθ4, filtered and concentrated under reduced pressure. The residue was purified by normal phase flash chromatography on silica gel (5% MeOH-CF^C^, Rf = 0.35) to provide 3- cyclopropyl-l-methyl-4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)-lH-pyrazole (294 mg, 48%) as a yellow oil.
References:
WO2010/141273,2010,A1 Location in patent:Page/Page column 37
765-43-5
401 suppliers
$10.00/1g

1257637-82-3
12 suppliers
inquiry
1053163-67-9
12 suppliers
$503.64/5MG

1257637-82-3
12 suppliers
inquiry

21666-68-2
38 suppliers
$57.50/250mg

1257637-82-3
12 suppliers
inquiry