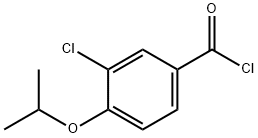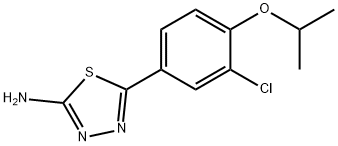
5-{3-chloro-4-[(1-Methylethyl)oxy]phenyl}-1,3,4-thiadiazol-2-aMine synthesis
- Product Name:5-{3-chloro-4-[(1-Methylethyl)oxy]phenyl}-1,3,4-thiadiazol-2-aMine
- CAS Number:1258440-61-7
- Molecular formula:C11H12ClN3OS
- Molecular Weight:269.75
Yield:1258440-61-7 45.6%
Reaction Conditions:
Stage #1: thiosemicarbazide;3-chloro-4-[(1-methylethyl)oxy]benzoyl chloridewith trichlorophosphate at 90; for 3 h;
Stage #2: with sodium hydroxide at 20;Cooling with ice;
Steps:
Phosphoric trichloride (17.41 g, 114 mmol) was added to a mixture of hydrazinecarbothioamide (5.17 g, 56.8 mmol) and 3-chloro-4-[(1- methylethyl)oxy]benzoyl chloride (14 g, 37.8 mmol) and the mixture was heated at 9O0C for 3 h. A sample was taken and quenched into a mixture of ice and 10M NaOH, then extracted with EtOAc. The heat was switched off and the mixture allowed to stand at room temperature overnight and then added to cold 5M NaOH solution, cooling in ice bath. The mixture was extracted with EtOAc (2 x 200 ml_), the solvent dried and evaporated to give a beige solid. Product was heated in ethanol (120 ml.) until it all dissolved, then cooled in an ice bath and the precipitated solid collected by filtration and dried in vacuo to give 5-{3-chloro-4-[(1- methylethyl)oxy]phenyl}-1 ,3,4-thiadiazol-2-amine (4.65 g, 45.6 %) 1 H NMR (DMSO-d6 ,400MHz): δ (ppm) 7.79 (d, J=2.0 Hz, 1 H), 7.63 (dd, J=QJ, 1.9 Hz, 1 H), 7.40 (s, 2H), 7.25 (d, J=8.6 Hz, 1 H), 4.75 (spt, J=5.9 Hz, 1 H), 1.32 (d, J=5.8 Hz, 6H)
References:
WO2010/146104,2010,A1 Location in patent:Page/Page column 25-26
213598-07-3
68 suppliers
$31.00/250mg

79-19-6
491 suppliers
$14.00/1g
![5-{3-chloro-4-[(1-Methylethyl)oxy]phenyl}-1,3,4-thiadiazol-2-aMine](/CAS/20150408/GIF/1258440-61-7.gif)
1258440-61-7
1 suppliers
inquiry

213598-07-3
68 suppliers
$31.00/250mg
![5-{3-chloro-4-[(1-Methylethyl)oxy]phenyl}-1,3,4-thiadiazol-2-aMine](/CAS/20150408/GIF/1258440-61-7.gif)
1258440-61-7
1 suppliers
inquiry