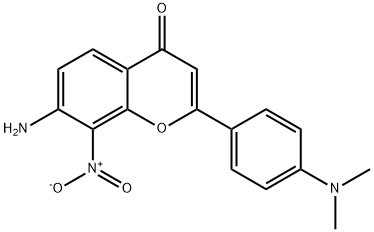
7-AMino-2-(4-(diMethylaMino)phenyl)-8-nitro-4H-chroMen-4-one synthesis
- Product Name:7-AMino-2-(4-(diMethylaMino)phenyl)-8-nitro-4H-chroMen-4-one
- CAS Number:1258637-96-5
- Molecular formula:C17H15N3O4
- Molecular Weight:325.32
1352289-21-4
0 suppliers
inquiry

1258637-96-5
6 suppliers
inquiry
Yield:1258637-96-5 89%
Reaction Conditions:
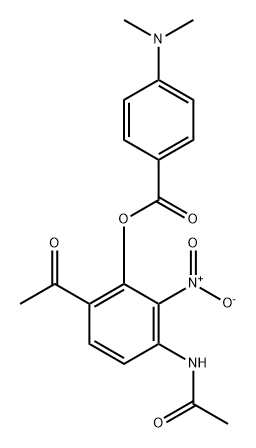
Stage #1: 3-acetamido-6-acetyl-2-nitrophenyl 4-(dimethylamino)benzoatewith pyridine;potassium hydroxide at 60; for 1 h;
Stage #2: with sulfuric acid;acetic acid at 110; for 0.5 h;
Steps:
Preparation of 7 -amino-2-( 4-( dimethylamino )phenyl)-8-nitro-4H-chromen-4-one ( 4)
A mixture of 3-acetamido-6-acetyl-2-nitrophenyl 4-( dimethylamino )benzoate (3, 2g, 1.0 eq.) and potassium hydroxide (8 g, 2.0 eq.) in pyridine (20 mL) was heated to 60°Cfor 1 hand poured into icy IN HCl (100 mL). The yellow solid was collected and10 dissolved in acetic acid (20 mL) and concentrated sulfuric acid. The resulting mixture washeated to llOOC for 30 min. The mixture was cooled tort and poured into saturatedsodium carbonate. The yellow solid was filtered and dried in vacuo to afford 7-amino-2-(4-(dimethylamino)phenyl)-8-nitro-4H-chromen-4-one (4) (1.5 g, yield: 89%)
References:
WO2014/18741,2014,A1 Location in patent:Page/Page column 35

30192-48-4
17 suppliers
inquiry

1258637-96-5
6 suppliers
inquiry
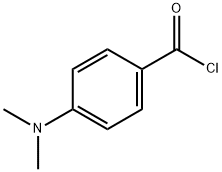
4755-50-4
112 suppliers
$8.00/1g

1258637-96-5
6 suppliers
inquiry