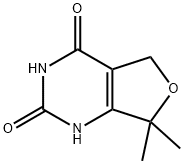
7,7-DiMethyl-1H,2H,3H,4H,5H,7H-furo[3,4-d]pyriMidine-2,4-dione synthesis
- Product Name:7,7-DiMethyl-1H,2H,3H,4H,5H,7H-furo[3,4-d]pyriMidine-2,4-dione
- CAS Number:1260088-71-8
- Molecular formula:C8H10N2O3
- Molecular Weight:182.18
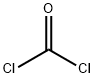
75-44-5
2 suppliers
inquiry
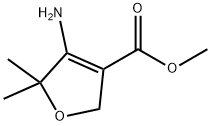
1260088-70-7
3 suppliers
inquiry
![7,7-DiMethyl-1H,2H,3H,4H,5H,7H-furo[3,4-d]pyriMidine-2,4-dione](/CAS/GIF/1260088-71-8.gif)
1260088-71-8
12 suppliers
$346.00/250mg
Yield:1260088-71-8 71%
Reaction Conditions:
with pyridine in 1,2-dichloro-ethane;toluene at 0; for 3 h;
Steps:
7.2
Step 2-Synthesis of o: To a cool (0° C.) solution of methyl 4-amino-5,5-dimethyl-2,5-dihydrofuran-3-carboxylate (n)(12.34 g, 72.1 mmol), pyridine (23.3 mL, 288 mmol) and 1,2-dichloroethane (250 mL) was added phosgene (20% solution in toluene, 50 mL, 86.5 mmol) in one portion. The mixture was maintained at 0° C. for 3 h, then 28% NH4OH (80 mL) was added in one portion and the mixture was stirred gently for 3 h, then heated at 50° C. for 16 h. Water (200 mL) was added and the phases separated. The organic phase was extracted with 1% NH4OH (2×100 mL). The combined aq. phases were washed with dichloromethane (3×20 mL), and concentrated to approximately 150 mL, which caused the product to precipitate. The ppt. was collected on paper, rinsed with water, and dried under high vacuum to afford 8.27 g of 7,7-dimethyl-5,7-dihydrofuro[3,4-d]pyrimidine-2,4(1H,3H)-dione (o) as colorless crystals, further concentration of the mother liquor provided a second crop of product 1.07 g (71% combined yield): 1H NMR (400 MHz, DMSO) δ 11.37 (s, 1H), 11.00 (m, 1H), 4.73 (s, 2H), 1.30 (s, 6H); LC-MS: m/z=+182 (M+H)+.
References:
US2010/331305,2010,A1 Location in patent:Page/Page column 32-33