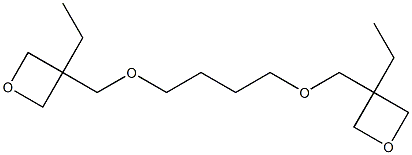
Oxetane, 3,3'-[1,4-butanediylbis(oxymethylene)]bis[3-ethyl- synthesis
- Product Name:Oxetane, 3,3'-[1,4-butanediylbis(oxymethylene)]bis[3-ethyl-
- CAS Number:126050-33-7
- Molecular formula:C16H30O4
- Molecular Weight:286.407
3893-75-2
0 suppliers
inquiry
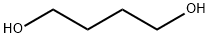
110-63-4
697 suppliers
$24.80/250g
![1-Butanol, 4-[(3-ethyl-3-oxetanyl)methoxy]-](/CAS/20200515/GIF/889867-34-9.gif)
889867-34-9
0 suppliers
inquiry
![Oxetane, 3,3'-[1,4-butanediylbis(oxymethylene)]bis[3-ethyl-](/CAS/20180528/GIF/126050-33-7.gif)
126050-33-7
2 suppliers
inquiry
Yield:889867-34-9 67% ,126050-33-7 7 %Chromat.
Reaction Conditions:
Stage #1: Butane-1,4-diolwith sodium hydroxide;tetrabutylammomium bromide in toluene at 60 - 75;
Stage #2: (3-ethyloxetan-3-yl)methyl methanesulfonate in toluene at 75 - 85; for 2 h;Product distribution / selectivity;
Steps:
1
Example 1 (Synthesis of 3-ethyl-3-(4-hydroxybutyl)oxymethyloxetane (hereinafter referred to as "HBOX")); 1,4-Butanediol (721 g (8.0 mol)) and toluene (350 ml) were added to a glass flask having an inner volume of 2,000 ml equipped with a stirrer, a thermometer, a dropping funnel and an reflux condenser, and mixture was warmed to 60°C with stirring. Tetrabutylammonium bromide (38.7 g (0.12 mol)) and 96% sodium hydroxide (184 g (4.4 mol)) were added, and the mixture warmed to 75°C with stirring. Subsequently 3-ethyl-3-methanesulfonyloxymethyloxetane (777 g (3.8 mol)) having purity of 95% synthesized by the same method as in Reference Example 1 was dropwise added while keeping a liquid temperature at 75-85°C, and the mixture was reacted at the same temperature for 2 hours. After the completion of the reaction, water (800 ml) was added to the reaction liquid (a bisoxetane ether compound generated in the amount of only 7% (an analysis value by a gas chromatography)), and a liquid separation was performed to give an organic layer. Toluene (800 ml) and water (400 ml) were added to a resultant organic layer, and acetic acid was added with stirring so that pH was adjusted to 9.5. After a liquid separation, an aqueous layer was extracted with toluene (400 ml) twice. An extract (a toluene layer) and an organic layer were combined, and the resultant was concentrated under reduced pressure. A resultant concentrate was distilled under reduced pressure (157-159°C, 1.9 kPa) to give, as a colorless liquid, 3-ethyl-3-(4-hydroxybutyl)oxymethyloxetane having a purity of 96% (an analysis value by a gas chromatography) (497 g) (an isolated yield based on 3-ethyl-3-methanesulfonyloxymethyloxetane: 67%). 3-Ethyl-3-(4-hydroxybutyl)oxymethyloxetane was a novel compound having the following physical properties. CI-MS (m/e) ; 189 (M+1) 1H-NMR (CDCl3, δ (ppm)) ; 0.88 (3H, t, J = 7.5), 1.58-1.68 (4H, m), 1.74 (2H, q, J = 7.5), 3.47-3.60 (7H, m), 4.34 (2H, d, J = 5.8), 4.44 (2H, d, J = 5.8)
References:
EP2030972,2009,A1 Location in patent:Page/Page column 6